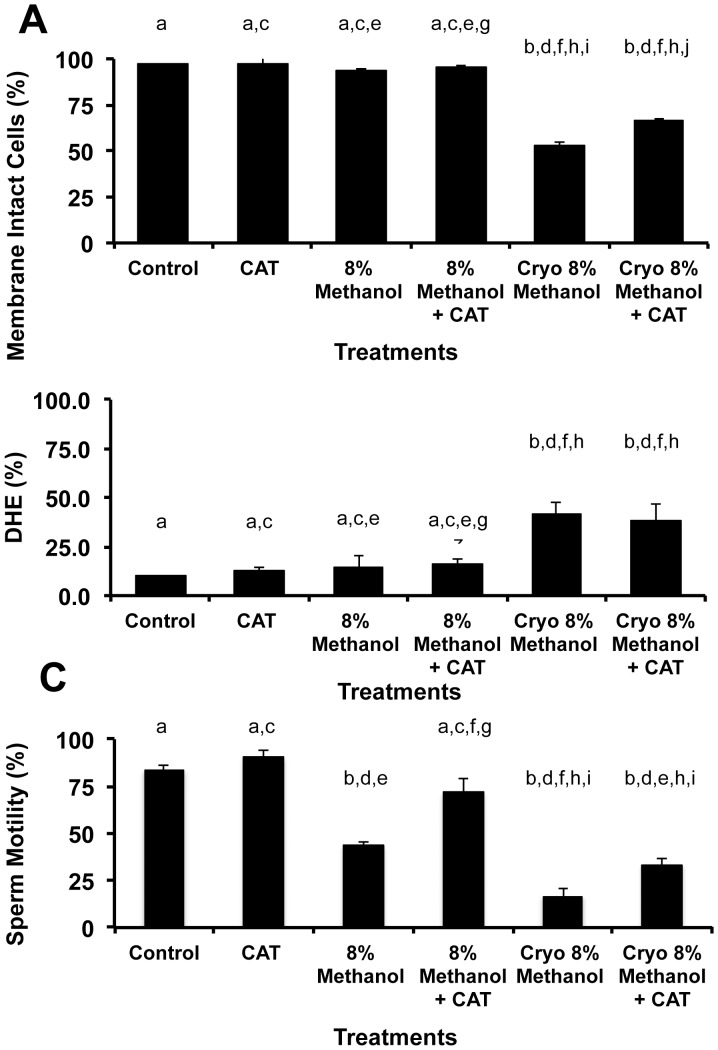
Figure 3. Evaluation of the intracellular ROS in zebrafish spermatozoa during cryopreservation and its effect on viability and motility.
(A) Overall, cryopreservation decreased the viability of zebrafish sperm by approximately 50%, but the addition of CAT to the methanol improved its post-thaw viability 16% (P<0.05, ANOVA and Tukey’s Multiple Comparison test, F = 60.5). (B) Cryopreservation increased ROS over 2.7-fold (P<0.05, ANOVA, and Tukey’s Multiple Comparison test F = 6.2), but the addition of CAT did not mitigate it (P>0.5, ANOVA). (C) Motility demonstrated the greatest sensitivity in our treatments. Prior to cryopreservation, the 8% methanol treatment reduced motility by ∼50%, and the addition of CAT to the 8% methanol returned the motility to control levels. In parallel, the cryopreserved sperm (8% methanol) decreased the pot-thaw motility of zebrafish sperm from 83% (control) to 17%, however the addition of CAT doubled this post-thaw motility (P<0.05, ANOVA and Tukey’s Multiple Comparison test, F = 22.6). Bars with the same letter are not significantly different (P>0.05), but bars with different letters are (P<0.05). X- axis categories and sample sizes were: Control = live sperm stained with DHE, N = 13; CAT (200 U/ml, concentration used in all CAT treatments), N = 9; 8% Methanol, N = 12; 8% Methanol + CAT 200, N = 12; Cryo 8% Methanol = sperm cryopreserved according to [6] with 8% methanol, N = 13; Cryo 8% Methanol + CAT, N = 13.