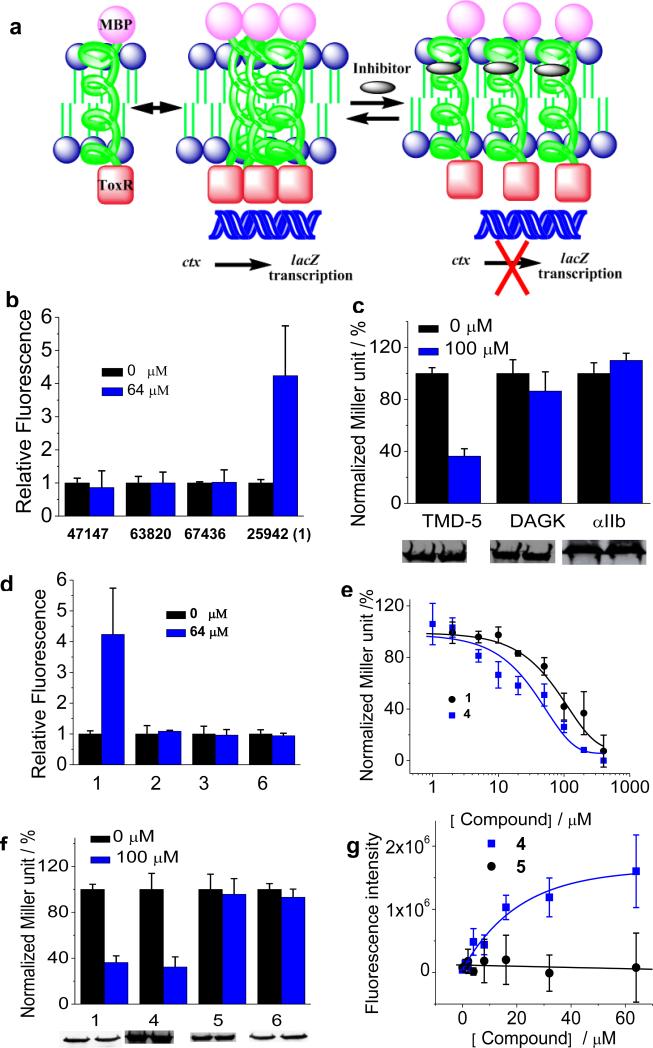
Figure 3.
(a), Schematic representation of the ToxR assay used for screening LMP-1 TMD-5 disruptors. (b), Coumarin fluorescence dequeching assay. Compounds were added into 100 nM of coumarin-labeled TMD-5 solution (50 mM HEPES, 150 μM C14 betaine, pH = 7.4) and equilibrated overnight. Samples were excited at 360 nm and emission was read at 430 nm using a Beckman-Coulter DTX 880 Multimode Detector plate reader. The background correction and the fluorescence of inhibitors were subtracted from the observed coumarin fluorescence signal. The fluorescence intensity of control sample (no compound) was set as 1.0. (c), ToxR measurement of the inhibitory effect of compound 1 on the oligomerization of LMP-1 TMD-5 and TMDs of diacylglycerol kinase (DAGK) and integrin αIIb. TMD oligomerization activity in the absence of compound was normalized as 100%. Western blot showed the chimeric MBP-TMD-ToxR protein expression level and gel loading normalized by OD600 nm of cultures. (d), Fluorescence dequenching assay of compound 1 and its analogues. Experiments were carried out as described in (b). (e), Dose-dependent titration of 1 and 4 on TMD-5 oligomerization using the ToxR assay. (f), ToxR measurement of oligomerization of TMD-5 in the presence of compound 1 or its analogues. Western blot showed the chimeric MBP-TMD-5-ToxR protein expression level and gel loading normalized by OD600 nm of cultures. (g), the dose dependent fluorescence enhancement curves of coumarin-labeled TMD-5 induced by compound 4 and compound 5. Experiments were carried out as described in (b).