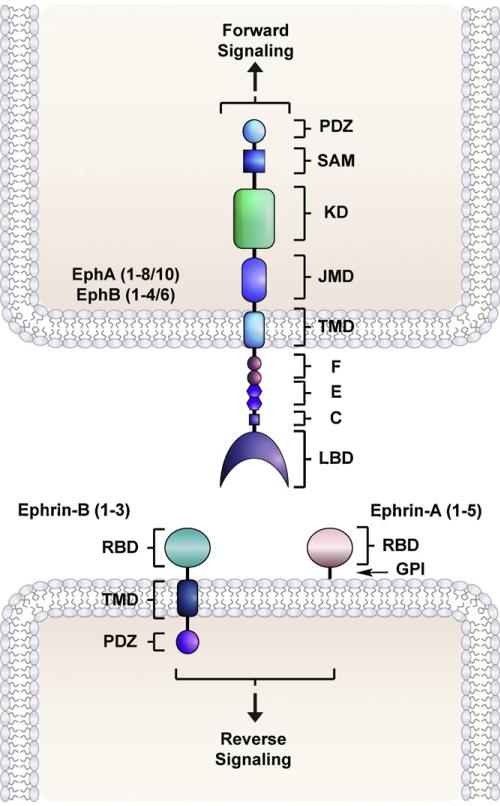
Fig. 1.
Eph receptors and ephrin ligands mediate cell–cell communication. Eph receptor tyrosine kinases interact with ephrin ligands residing on adjacent cell surfaces. Humans possess nine EphA (1–8/10) and five EphB (1–4/6) receptor sub-types, five ephrin-A (1–5) and three ephrin-B (1–3) ligands. Eph receptors are type I transmembrane spanning proteins that consist of a ligand binding domain (LBD), a cysteine-rich region (C), an epidermal growth factor (EGF)-like motif (E), fibronectin type-III repeats (F), a transmembrane domain (TMD), a juxtamembrane domain (JMD), a kinase domain (KD), a sterile-α-motif (SAM)-domain, and a PSD95/Dlg/ZO1 (PDZ)- protein binding site. Both subclasses of ephrin ligands consist of an N-terminal receptor binding domain (RBD). However, ephrin-A ligands are glycosylphosphatidylinositol (GPI)-linked whereas ephrin-B ligands are trans-membrane proteins and contain a cytoplasmic PDZ-tail. Upon engaging ephrins, bidirectional signaling is initiated where forward and reverse signaling take place in the receptor and ligand expressing cell, respectively.