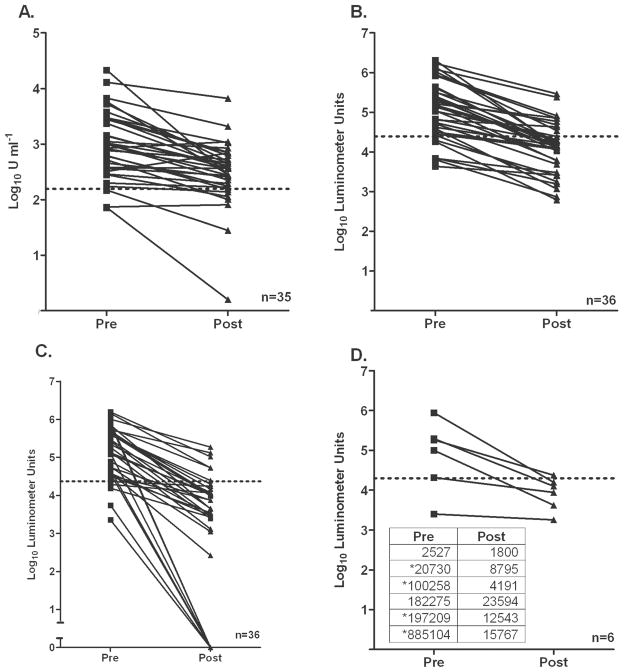
Figure 4.
Antibody titers before and after treatment for Strongyloides stercoralis infection, as determined by NIE ELISA (A), the NIE luciferase immunoprecipitation systems (LIPS) assay (B), the S. stercoralis immunoreactive antigen (SsIR) LIPS assay (C), and the SsIR/NIE LIPS assay (D). A significant difference was found between antibody levels at baseline and at the last follow-up time point (for the NIE ELISA, P < .0017; for the NIE LIPS assay, P < .0001; for the SsIR LIPS assay, P < .0001; for the SsIR/NIE LIPS assay, P < .0001; Mann-Whitney U test). Dotted lines indicate arbitrary cutoff values determined for each assay. Seroreversion was seen for 58% (21/36) of patients by the NIE LIPS assay, on the basis of a cutoff of 24,772 LU; 17% (6/35) of patients by the NIE ELISA, on the basis of a cutoff of 156 U mL−1; and 69% (25/36) of patients by the Ruc-SsIR assay, on the basis of a cutoff of 23,565 LU. The mean duration of follow-up for 36 patients was 17.47 months (range, 6 –32 months). A combination assay, Ruc-SsIR/Ruc-NIE, was tested in 6 patients who did not serorevert by either the NIE LIPS or SsIR LIPS assay (D). Four of 6 selected patients tested by the Ruc-SsIR/Ruc-NIE assay demonstrated seroreversion on the basis of a cutoff value of 20,000 LU. Pre- and posttreatment titers for each patient are shown in the inset in panel D. Asterisks indicate samples demonstrating seroreversion.