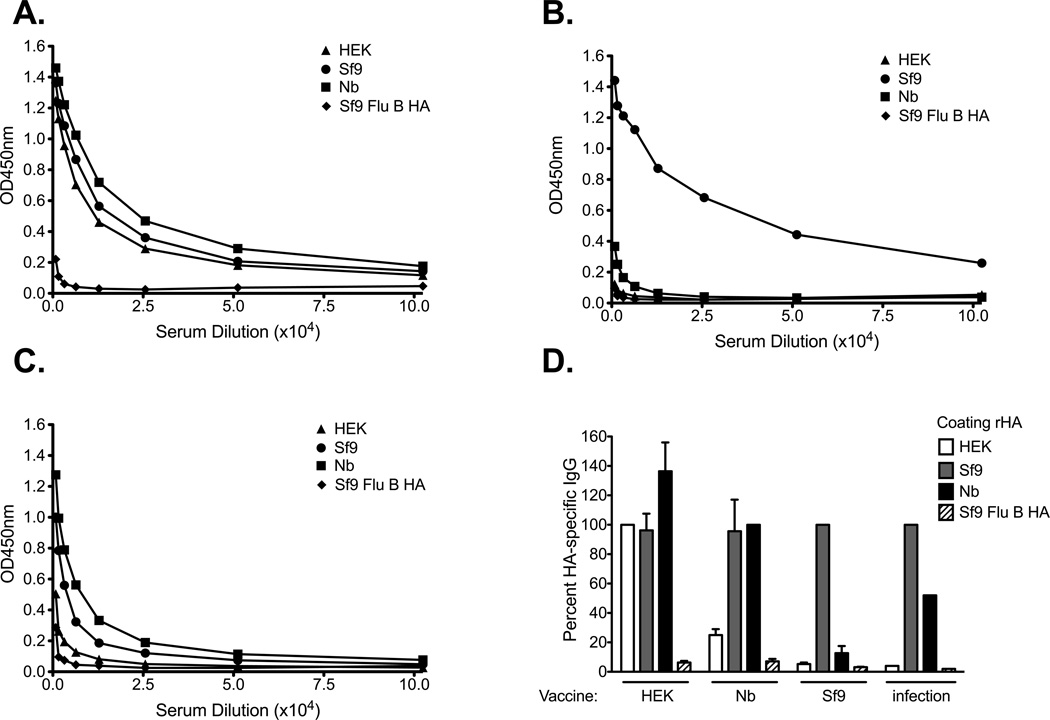
Figure 5. Recombinant HA IgG binding profile following vaccination with various rHAs from A/Brisbane/59/07.
B6 female mice were immunized intramuscularly (left flank) with 5ug of rHA adjuvanted with CpG. 14 days after priming, the mice received a booster immunization consisting of the same rHA preparation (5µg rHA and CpG). Serum from mice immunized with (A) HEK-, (B) Sf9-, or (C) Nb- derived HA was collected 21 days after boost. ELISA plates were coated overnight with equal concentrations of each of the rHA proteins (0.05ug/well). Serum was diluted two-fold and added to the rHA-coated plates. A representative plot for one mouse is presented in panels A–C. In panel D the antibody binding was normalized against the rHA administered as vaccine. The percentage of IgG detected to each version of the rHA protein is plotted in panel D. Error bars represent the standard error of the mean for n= 3–4 mice/group. Pooled convalescent serum from A/Brisbane/59/07 was normalized against the Sf9-derived HA and is presented as a control (D). The proportion of IgG detected to each version of the rHA protein is plotted in panel D.