Abstract
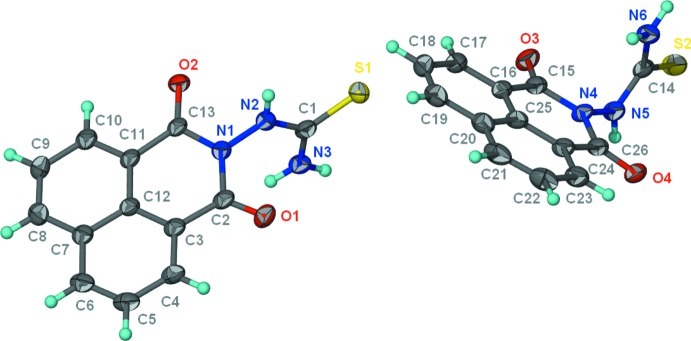
In the two independent molecules in the asymmetric unit of the title compound, C13H9N3O2S, the azatricyclotridecapentaene ring system is approximately planar with r.m.s. deviations of 0.022 and 0.033 Å. The urea unit connected to the fused rings is approximately perpendicular [dihedral angles = 82.4 (1) and 82.7 (1)°]. In the crystal, the molecules associate by N—H⋯O hydrogen bonds, forming a chain running along the a axis. The crystal studied was a non-merohedral twin with a fractional contribution of 49.6 (1)% for the minor domain.
Related literature
For background to the class of antitumor drugs, see; Pessoa et al. (2010 ▶).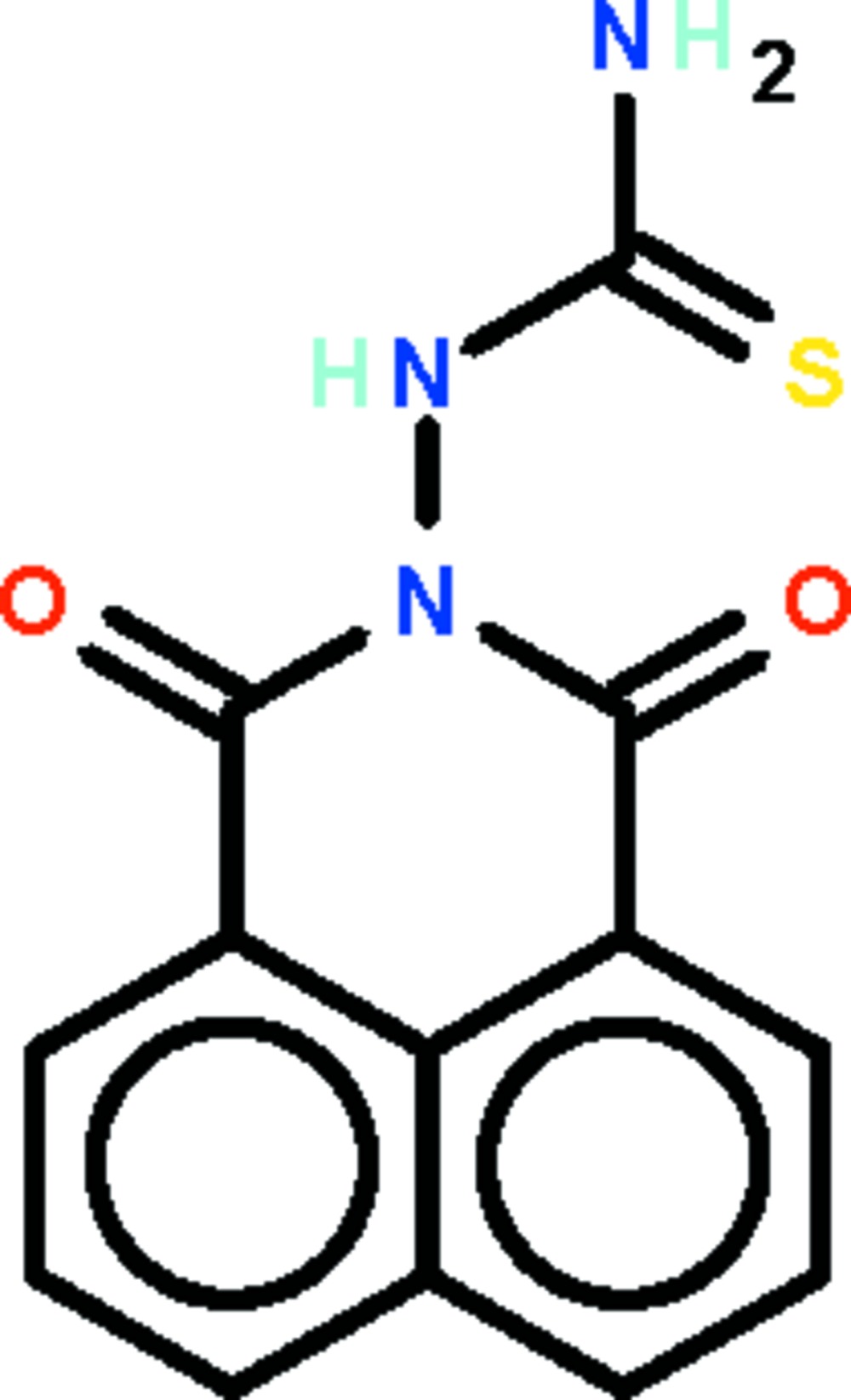
Experimental
Crystal data
C13H9N3O2S
M r = 271.29
Triclinic,
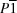
a = 4.5861 (2) Å
b = 11.0475 (4) Å
c = 22.5594 (9) Å
α = 89.196 (3)°
β = 88.073 (3)°
γ = 81.128 (3)°
V = 1128.61 (8) Å3
Z = 4
Cu Kα radiation
μ = 2.58 mm−1
T = 100 K
0.25 × 0.10 × 0.03 mm
Data collection
Agilent SuperNova Dual diffractometer with an Atlas detector
Absorption correction: multi-scan (CrysAlis PRO; Agilent, 2012 ▶) T min = 0.565, T max = 0.927
14869 measured reflections
7974 independent reflections
7060 reflections with I > 2σ(I)
R int = 0.076
Refinement
R[F 2 > 2σ(F 2)] = 0.065
wR(F 2) = 0.196
S = 1.11
7974 reflections
345 parameters
H-atom parameters constrained
Δρmax = 0.47 e Å−3
Δρmin = −0.73 e Å−3
Data collection: CrysAlis PRO (Agilent, 2012 ▶); cell refinement: CrysAlis PRO; data reduction: CrysAlis PRO; program(s) used to solve structure: SHELXS97 (Sheldrick, 2008 ▶); program(s) used to refine structure: SHELXL97 (Sheldrick, 2008 ▶); molecular graphics: X-SEED (Barbour, 2001 ▶); software used to prepare material for publication: publCIF (Westrip, 2010 ▶).
Supplementary Material
Crystal structure: contains datablock(s) global, I. DOI: 10.1107/S1600536812021812/bt5920sup1.cif
Structure factors: contains datablock(s) I. DOI: 10.1107/S1600536812021812/bt5920Isup2.hkl
Supplementary material file. DOI: 10.1107/S1600536812021812/bt5920Isup3.cml
Additional supplementary materials: crystallographic information; 3D view; checkCIF report
Table 1. Hydrogen-bond geometry (Å, °).
| D—H⋯A | D—H | H⋯A | D⋯A | D—H⋯A |
|---|---|---|---|---|
| N2—H2⋯O1i | 0.88 | 2.35 | 3.074 (3) | 140 |
| N2—H2⋯O1ii | 0.88 | 2.33 | 2.993 (3) | 132 |
| N3—H31⋯O2iii | 0.88 | 2.09 | 2.912 (3) | 154 |
| N5—H5⋯O3iii | 0.88 | 2.55 | 3.301 (3) | 144 |
| N5—H5⋯O3iv | 0.88 | 2.52 | 2.988 (3) | 114 |
| N6—H61⋯O4i | 0.88 | 2.21 | 3.015 (3) | 152 |
Symmetry codes: (i) 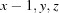 ; (ii)
; (ii) 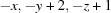 ; (iii)
; (iii) 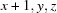 ; (iv)
; (iv) 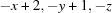 .
.
Acknowledgments
We thank the Research Center of the College of Pharmacy College and Deanship of Scientific Research of King Saud University, and the Ministry of Higher Education of Malaysia (grant No. UM.C/HIR/MOHE/SC/12) for supporting this study.
supplementary crystallographic information
Comment
1-(1,3-Dioxo-1,3-dihydro-2H-isoindol-2-yl)thiourea is a powerful antitumor drug that is synthesized from phthalic anhydride and thiosemicarbazide (Pessoa et al., 2010). The title compound (Scheme I) is synthesized by using naphthalic acid anhydride in place of phthalic anhydride.
The crystal structure features two independent molecules. The azatricyclo-trideca-pentaene fused-ring is flat; the urea unit connected to the fused-ring is approximately perpendicular [dihedral angle 82.4 (1), 82.7 (1) °] (Fig. 1). Each independent molecule associates by an N–H···O hydrogen bond into a dimer; adjacent dimers are linked by N–H···O hydrogen bonds to form a chain running along the a-axis of the triclinic unit cell (Table 1).
Experimental
Naphthalic acid anhydride (2.2 mmol, 0.44 g) was dissolved in glacial acetic acid (15 ml). A solution of thiosemicarbazide (2.8 mmol, 0.26 g) in glacial acetic acid (10 ml) was added. A solid formed immediately. The solid was collected, washed with water and ether. The compound was recrystallized from a mixture of toluene and DMF to yield colorless crystals.
Refinement
Hydrogen atoms were placed in calculated positions [C–H 0.95, N–H 0.88 Å, Uiso(H) 1.2Ueq(C,N)] and were included in the refinement in the riding model approximation.
The crystal is a non-merohedral twin with a minor compound being of 49.6 (1)%.
The somewhat large weighting scheme is probably an artifact of twinning.
Figures
Fig. 1.
Anisotropic displacement ellipsoid plot (Barbour, 2001) of the two independent molecules of C13H9N3O2S at the 70% probability level; hydrogen atoms are drawn as spheres of arbitrary radius.
Crystal data
| C13H9N3O2S | Z = 4 |
| Mr = 271.29 | F(000) = 560 |
| Triclinic, P1 | Dx = 1.597 Mg m−3 |
| Hall symbol: -P 1 | Cu Kα radiation, λ = 1.54184 Å |
| a = 4.5861 (2) Å | Cell parameters from 6016 reflections |
| b = 11.0475 (4) Å | θ = 3.9–76.4° |
| c = 22.5594 (9) Å | µ = 2.58 mm−1 |
| α = 89.196 (3)° | T = 100 K |
| β = 88.073 (3)° | Plate, colorless |
| γ = 81.128 (3)° | 0.25 × 0.10 × 0.03 mm |
| V = 1128.61 (8) Å3 |
Data collection
| Agilent SuperNova Dual diffractometer with an Atlas detector | 7974 independent reflections |
| Radiation source: SuperNova (Cu) X-ray Source | 7060 reflections with I > 2σ(I) |
| Mirror monochromator | Rint = 0.076 |
| Detector resolution: 10.4041 pixels mm-1 | θmax = 77.5°, θmin = 3.9° |
| ω scan | h = −5→5 |
| Absorption correction: multi-scan (CrysAlis PRO; Agilent, 2012) | k = −13→13 |
| Tmin = 0.565, Tmax = 0.927 | l = −28→24 |
| 14869 measured reflections |
Refinement
| Refinement on F2 | Secondary atom site location: difference Fourier map |
| Least-squares matrix: full | Hydrogen site location: inferred from neighbouring sites |
| R[F2 > 2σ(F2)] = 0.065 | H-atom parameters constrained |
| wR(F2) = 0.196 | w = 1/[σ2(Fo2) + (0.1312P)2 + 0.6561P] where P = (Fo2 + 2Fc2)/3 |
| S = 1.11 | (Δ/σ)max = 0.001 |
| 7974 reflections | Δρmax = 0.47 e Å−3 |
| 345 parameters | Δρmin = −0.73 e Å−3 |
| 0 restraints | Extinction correction: SHELXL97 (Sheldrick, 2008), Fc*=kFc[1+0.001xFc2λ3/sin(2θ)]-1/4 |
| Primary atom site location: structure-invariant direct methods | Extinction coefficient: 0.007 (1) |
Fractional atomic coordinates and isotropic or equivalent isotropic displacement parameters (Å2)
| x | y | z | Uiso*/Ueq | ||
| S1 | −0.00401 (14) | 0.90107 (6) | 0.35711 (3) | 0.02668 (18) | |
| S2 | 1.03964 (15) | 0.64485 (7) | −0.15038 (3) | 0.03115 (19) | |
| O1 | 0.3711 (4) | 0.91572 (19) | 0.53190 (8) | 0.0280 (4) | |
| O2 | −0.2323 (4) | 0.63074 (18) | 0.50792 (8) | 0.0247 (4) | |
| O3 | 0.6884 (5) | 0.5699 (2) | 0.02003 (9) | 0.0315 (4) | |
| O4 | 1.2207 (4) | 0.88347 (18) | 0.01639 (9) | 0.0268 (4) | |
| N1 | 0.0726 (5) | 0.7727 (2) | 0.52057 (9) | 0.0235 (4) | |
| N2 | −0.0298 (5) | 0.8305 (2) | 0.46861 (10) | 0.0243 (5) | |
| H2 | −0.2080 | 0.8736 | 0.4689 | 0.029* | |
| N3 | 0.3931 (5) | 0.7492 (2) | 0.41554 (10) | 0.0255 (5) | |
| H31 | 0.4532 | 0.7066 | 0.4473 | 0.031* | |
| H32 | 0.5037 | 0.7429 | 0.3828 | 0.031* | |
| N4 | 0.9302 (5) | 0.7334 (2) | 0.01767 (9) | 0.0231 (4) | |
| N5 | 1.0573 (5) | 0.6935 (2) | −0.03728 (10) | 0.0248 (5) | |
| H5 | 1.2422 | 0.6574 | −0.0398 | 0.030* | |
| N6 | 0.6345 (5) | 0.7835 (2) | −0.08405 (10) | 0.0288 (5) | |
| H61 | 0.5714 | 0.8196 | −0.0505 | 0.035* | |
| H62 | 0.5260 | 0.7957 | −0.1156 | 0.035* | |
| C1 | 0.1363 (6) | 0.8228 (2) | 0.41709 (11) | 0.0231 (5) | |
| C2 | 0.2727 (5) | 0.8289 (3) | 0.55335 (11) | 0.0237 (5) | |
| C3 | 0.3503 (5) | 0.7740 (2) | 0.61137 (11) | 0.0228 (5) | |
| C4 | 0.5301 (6) | 0.8282 (3) | 0.64730 (12) | 0.0257 (5) | |
| H4 | 0.6002 | 0.9012 | 0.6347 | 0.031* | |
| C5 | 0.6094 (6) | 0.7749 (3) | 0.70274 (12) | 0.0288 (6) | |
| H5A | 0.7324 | 0.8127 | 0.7274 | 0.035* | |
| C6 | 0.5111 (6) | 0.6693 (3) | 0.72147 (12) | 0.0277 (6) | |
| H6 | 0.5666 | 0.6347 | 0.7590 | 0.033* | |
| C7 | 0.3271 (5) | 0.6110 (3) | 0.68538 (11) | 0.0241 (5) | |
| C8 | 0.2256 (6) | 0.5010 (3) | 0.70251 (12) | 0.0264 (5) | |
| H8 | 0.2803 | 0.4639 | 0.7396 | 0.032* | |
| C9 | 0.0490 (6) | 0.4467 (3) | 0.66639 (12) | 0.0258 (5) | |
| H9 | −0.0141 | 0.3718 | 0.6783 | 0.031* | |
| C10 | −0.0390 (6) | 0.5017 (3) | 0.61165 (12) | 0.0255 (5) | |
| H10 | −0.1647 | 0.4649 | 0.5872 | 0.031* | |
| C11 | 0.0577 (5) | 0.6086 (2) | 0.59394 (11) | 0.0222 (5) | |
| C12 | 0.2453 (5) | 0.6650 (2) | 0.62956 (11) | 0.0224 (5) | |
| C13 | −0.0487 (5) | 0.6671 (2) | 0.53792 (11) | 0.0222 (5) | |
| C14 | 0.8931 (6) | 0.7108 (3) | −0.08703 (12) | 0.0254 (5) | |
| C15 | 0.7413 (6) | 0.6591 (3) | 0.04568 (12) | 0.0254 (5) | |
| C16 | 0.6217 (6) | 0.6980 (3) | 0.10479 (12) | 0.0249 (5) | |
| C17 | 0.4327 (6) | 0.6302 (3) | 0.13435 (13) | 0.0294 (6) | |
| H17 | 0.3849 | 0.5583 | 0.1169 | 0.035* | |
| C18 | 0.3116 (6) | 0.6675 (3) | 0.19013 (13) | 0.0330 (6) | |
| H18 | 0.1787 | 0.6215 | 0.2100 | 0.040* | |
| C19 | 0.3827 (6) | 0.7698 (3) | 0.21630 (13) | 0.0325 (6) | |
| H19 | 0.3023 | 0.7928 | 0.2546 | 0.039* | |
| C20 | 0.5755 (6) | 0.8421 (3) | 0.18675 (12) | 0.0286 (6) | |
| C21 | 0.6513 (6) | 0.9491 (3) | 0.21190 (13) | 0.0328 (6) | |
| H21 | 0.5746 | 0.9737 | 0.2502 | 0.039* | |
| C22 | 0.8338 (7) | 1.0178 (3) | 0.18183 (14) | 0.0341 (6) | |
| H22 | 0.8777 | 1.0907 | 0.1990 | 0.041* | |
| C23 | 0.9566 (6) | 0.9808 (3) | 0.12557 (12) | 0.0295 (6) | |
| H23 | 1.0853 | 1.0281 | 0.1052 | 0.035* | |
| C24 | 0.8896 (6) | 0.8757 (3) | 0.10003 (12) | 0.0249 (5) | |
| C25 | 0.6951 (6) | 0.8056 (3) | 0.12967 (12) | 0.0258 (5) | |
| C26 | 1.0303 (6) | 0.8364 (3) | 0.04201 (12) | 0.0243 (5) |
Atomic displacement parameters (Å2)
| U11 | U22 | U33 | U12 | U13 | U23 | |
| S1 | 0.0238 (3) | 0.0370 (3) | 0.0193 (3) | −0.0052 (2) | 0.0006 (2) | 0.0020 (2) |
| S2 | 0.0249 (3) | 0.0490 (4) | 0.0204 (3) | −0.0084 (3) | 0.0019 (2) | −0.0066 (3) |
| O1 | 0.0271 (9) | 0.0336 (10) | 0.0251 (10) | −0.0113 (8) | 0.0060 (7) | −0.0019 (8) |
| O2 | 0.0200 (9) | 0.0343 (10) | 0.0213 (9) | −0.0089 (7) | −0.0012 (7) | −0.0016 (7) |
| O3 | 0.0315 (10) | 0.0392 (11) | 0.0280 (10) | −0.0190 (8) | 0.0005 (8) | −0.0043 (8) |
| O4 | 0.0201 (8) | 0.0343 (10) | 0.0275 (9) | −0.0092 (7) | 0.0010 (7) | −0.0001 (8) |
| N1 | 0.0203 (10) | 0.0334 (11) | 0.0179 (10) | −0.0078 (9) | 0.0001 (8) | 0.0010 (9) |
| N2 | 0.0177 (10) | 0.0338 (11) | 0.0210 (10) | −0.0037 (8) | 0.0010 (8) | 0.0030 (9) |
| N3 | 0.0182 (10) | 0.0368 (12) | 0.0216 (11) | −0.0051 (9) | 0.0017 (8) | −0.0020 (9) |
| N4 | 0.0168 (9) | 0.0354 (11) | 0.0184 (10) | −0.0090 (8) | 0.0036 (8) | −0.0053 (9) |
| N5 | 0.0195 (10) | 0.0359 (12) | 0.0195 (10) | −0.0066 (8) | 0.0028 (8) | −0.0043 (9) |
| N6 | 0.0195 (10) | 0.0457 (13) | 0.0212 (11) | −0.0053 (9) | 0.0004 (8) | −0.0001 (10) |
| C1 | 0.0198 (11) | 0.0286 (12) | 0.0227 (12) | −0.0097 (9) | 0.0007 (9) | −0.0034 (10) |
| C2 | 0.0172 (11) | 0.0340 (13) | 0.0209 (12) | −0.0079 (10) | 0.0050 (9) | −0.0040 (10) |
| C3 | 0.0169 (11) | 0.0310 (12) | 0.0207 (12) | −0.0053 (9) | 0.0029 (9) | −0.0031 (10) |
| C4 | 0.0193 (11) | 0.0338 (13) | 0.0254 (13) | −0.0083 (10) | 0.0019 (10) | −0.0050 (10) |
| C5 | 0.0202 (12) | 0.0404 (15) | 0.0266 (13) | −0.0065 (11) | −0.0012 (10) | −0.0081 (11) |
| C6 | 0.0189 (12) | 0.0444 (16) | 0.0199 (12) | −0.0047 (11) | 0.0004 (9) | −0.0052 (11) |
| C7 | 0.0184 (11) | 0.0326 (13) | 0.0212 (12) | −0.0044 (10) | 0.0029 (9) | −0.0014 (10) |
| C8 | 0.0223 (12) | 0.0344 (13) | 0.0212 (12) | −0.0014 (10) | 0.0038 (10) | 0.0021 (10) |
| C9 | 0.0259 (12) | 0.0283 (12) | 0.0236 (12) | −0.0066 (10) | 0.0052 (10) | 0.0013 (10) |
| C10 | 0.0223 (12) | 0.0342 (13) | 0.0210 (12) | −0.0086 (10) | 0.0034 (9) | −0.0025 (10) |
| C11 | 0.0181 (11) | 0.0306 (12) | 0.0188 (11) | −0.0078 (9) | 0.0040 (9) | −0.0017 (10) |
| C12 | 0.0174 (11) | 0.0303 (12) | 0.0204 (12) | −0.0066 (9) | 0.0009 (9) | −0.0003 (10) |
| C13 | 0.0163 (11) | 0.0310 (12) | 0.0201 (12) | −0.0071 (9) | 0.0042 (9) | −0.0010 (10) |
| C14 | 0.0197 (12) | 0.0358 (13) | 0.0229 (12) | −0.0121 (10) | 0.0015 (9) | −0.0007 (10) |
| C15 | 0.0201 (11) | 0.0351 (13) | 0.0224 (13) | −0.0083 (10) | −0.0025 (10) | −0.0006 (10) |
| C16 | 0.0183 (11) | 0.0365 (13) | 0.0208 (12) | −0.0074 (10) | −0.0006 (9) | 0.0018 (10) |
| C17 | 0.0211 (12) | 0.0403 (15) | 0.0276 (14) | −0.0080 (11) | −0.0008 (10) | 0.0057 (11) |
| C18 | 0.0205 (12) | 0.0484 (17) | 0.0293 (14) | −0.0048 (11) | 0.0031 (10) | 0.0120 (12) |
| C19 | 0.0228 (13) | 0.0507 (17) | 0.0218 (13) | 0.0007 (12) | 0.0019 (10) | 0.0040 (12) |
| C20 | 0.0216 (12) | 0.0405 (15) | 0.0220 (13) | 0.0002 (11) | −0.0009 (10) | 0.0007 (11) |
| C21 | 0.0269 (13) | 0.0474 (17) | 0.0217 (13) | 0.0027 (12) | −0.0036 (10) | −0.0064 (12) |
| C22 | 0.0346 (15) | 0.0380 (15) | 0.0288 (14) | −0.0008 (12) | −0.0082 (12) | −0.0079 (12) |
| C23 | 0.0271 (13) | 0.0381 (15) | 0.0242 (13) | −0.0069 (11) | −0.0036 (11) | −0.0028 (11) |
| C24 | 0.0213 (12) | 0.0332 (13) | 0.0212 (12) | −0.0068 (10) | −0.0016 (10) | −0.0035 (10) |
| C25 | 0.0174 (11) | 0.0388 (14) | 0.0214 (12) | −0.0045 (10) | −0.0017 (9) | −0.0006 (11) |
| C26 | 0.0189 (11) | 0.0344 (13) | 0.0207 (12) | −0.0074 (10) | −0.0019 (9) | −0.0010 (10) |
Geometric parameters (Å, º)
| S1—C1 | 1.687 (3) | C6—H6 | 0.9500 |
| S2—C14 | 1.685 (3) | C7—C8 | 1.411 (4) |
| O1—C2 | 1.210 (3) | C7—C12 | 1.423 (4) |
| O2—C13 | 1.216 (3) | C8—C9 | 1.374 (4) |
| O3—C15 | 1.210 (3) | C8—H8 | 0.9500 |
| O4—C26 | 1.210 (3) | C9—C10 | 1.412 (4) |
| N1—N2 | 1.388 (3) | C9—H9 | 0.9500 |
| N1—C13 | 1.413 (3) | C10—C11 | 1.374 (4) |
| N1—C2 | 1.417 (3) | C10—H10 | 0.9500 |
| N2—C1 | 1.364 (3) | C11—C12 | 1.414 (3) |
| N2—H2 | 0.8800 | C11—C13 | 1.475 (4) |
| N3—C1 | 1.324 (3) | C15—C16 | 1.469 (4) |
| N3—H31 | 0.8800 | C16—C17 | 1.380 (4) |
| N3—H32 | 0.8800 | C16—C25 | 1.413 (4) |
| N4—N5 | 1.399 (3) | C17—C18 | 1.400 (4) |
| N4—C15 | 1.412 (3) | C17—H17 | 0.9500 |
| N4—C26 | 1.414 (3) | C18—C19 | 1.370 (5) |
| N5—C14 | 1.367 (3) | C18—H18 | 0.9500 |
| N5—H5 | 0.8800 | C19—C20 | 1.423 (4) |
| N6—C14 | 1.326 (4) | C19—H19 | 0.9500 |
| N6—H61 | 0.8800 | C20—C21 | 1.414 (4) |
| N6—H62 | 0.8800 | C20—C25 | 1.422 (4) |
| C2—C3 | 1.466 (4) | C21—C22 | 1.372 (5) |
| C3—C4 | 1.381 (3) | C21—H21 | 0.9500 |
| C3—C12 | 1.415 (4) | C22—C23 | 1.411 (4) |
| C4—C5 | 1.409 (4) | C22—H22 | 0.9500 |
| C4—H4 | 0.9500 | C23—C24 | 1.384 (4) |
| C5—C6 | 1.371 (4) | C23—H23 | 0.9500 |
| C5—H5A | 0.9500 | C24—C25 | 1.415 (4) |
| C6—C7 | 1.421 (4) | C24—C26 | 1.480 (4) |
| N2—N1—C13 | 116.5 (2) | C10—C11—C12 | 121.2 (2) |
| N2—N1—C2 | 117.5 (2) | C10—C11—C13 | 118.6 (2) |
| C13—N1—C2 | 125.9 (2) | C12—C11—C13 | 120.1 (2) |
| C1—N2—N1 | 122.3 (2) | C11—C12—C3 | 121.7 (2) |
| C1—N2—H2 | 118.9 | C11—C12—C7 | 118.8 (2) |
| N1—N2—H2 | 118.9 | C3—C12—C7 | 119.4 (2) |
| C1—N3—H31 | 120.0 | O2—C13—N1 | 120.4 (2) |
| C1—N3—H32 | 120.0 | O2—C13—C11 | 123.9 (2) |
| H31—N3—H32 | 120.0 | N1—C13—C11 | 115.7 (2) |
| N5—N4—C15 | 116.5 (2) | N6—C14—N5 | 118.8 (2) |
| N5—N4—C26 | 116.2 (2) | N6—C14—S2 | 123.1 (2) |
| C15—N4—C26 | 126.9 (2) | N5—C14—S2 | 118.0 (2) |
| C14—N5—N4 | 119.8 (2) | O3—C15—N4 | 118.9 (2) |
| C14—N5—H5 | 120.1 | O3—C15—C16 | 124.9 (3) |
| N4—N5—H5 | 120.1 | N4—C15—C16 | 116.2 (2) |
| C14—N6—H61 | 120.0 | C17—C16—C25 | 121.2 (3) |
| C14—N6—H62 | 120.0 | C17—C16—C15 | 119.2 (3) |
| H61—N6—H62 | 120.0 | C25—C16—C15 | 119.7 (2) |
| N3—C1—N2 | 118.5 (2) | C16—C17—C18 | 119.9 (3) |
| N3—C1—S1 | 122.9 (2) | C16—C17—H17 | 120.1 |
| N2—C1—S1 | 118.5 (2) | C18—C17—H17 | 120.1 |
| O1—C2—N1 | 119.1 (2) | C19—C18—C17 | 120.7 (3) |
| O1—C2—C3 | 124.6 (2) | C19—C18—H18 | 119.7 |
| N1—C2—C3 | 116.3 (2) | C17—C18—H18 | 119.7 |
| C4—C3—C12 | 120.6 (2) | C18—C19—C20 | 120.8 (3) |
| C4—C3—C2 | 119.5 (2) | C18—C19—H19 | 119.6 |
| C12—C3—C2 | 119.9 (2) | C20—C19—H19 | 119.6 |
| C3—C4—C5 | 119.9 (3) | C21—C20—C25 | 118.8 (3) |
| C3—C4—H4 | 120.1 | C21—C20—C19 | 122.5 (3) |
| C5—C4—H4 | 120.1 | C25—C20—C19 | 118.7 (3) |
| C6—C5—C4 | 120.8 (2) | C22—C21—C20 | 121.1 (3) |
| C6—C5—H5A | 119.6 | C22—C21—H21 | 119.5 |
| C4—C5—H5A | 119.6 | C20—C21—H21 | 119.5 |
| C5—C6—C7 | 120.7 (3) | C21—C22—C23 | 120.3 (3) |
| C5—C6—H6 | 119.6 | C21—C22—H22 | 119.8 |
| C7—C6—H6 | 119.6 | C23—C22—H22 | 119.8 |
| C8—C7—C6 | 122.5 (2) | C24—C23—C22 | 120.0 (3) |
| C8—C7—C12 | 118.9 (2) | C24—C23—H23 | 120.0 |
| C6—C7—C12 | 118.6 (2) | C22—C23—H23 | 120.0 |
| C9—C8—C7 | 121.0 (2) | C23—C24—C25 | 120.4 (2) |
| C9—C8—H8 | 119.5 | C23—C24—C26 | 119.0 (2) |
| C7—C8—H8 | 119.5 | C25—C24—C26 | 120.6 (2) |
| C8—C9—C10 | 120.3 (2) | C16—C25—C24 | 121.8 (2) |
| C8—C9—H9 | 119.9 | C16—C25—C20 | 118.8 (3) |
| C10—C9—H9 | 119.9 | C24—C25—C20 | 119.4 (3) |
| C11—C10—C9 | 119.8 (2) | O4—C26—N4 | 120.4 (2) |
| C11—C10—H10 | 120.1 | O4—C26—C24 | 125.1 (2) |
| C9—C10—H10 | 120.1 | N4—C26—C24 | 114.5 (2) |
| C13—N1—N2—C1 | −105.9 (3) | C10—C11—C13—N1 | −176.4 (2) |
| C2—N1—N2—C1 | 78.2 (3) | C12—C11—C13—N1 | 6.3 (3) |
| C15—N4—N5—C14 | 77.9 (3) | N4—N5—C14—N6 | 11.6 (4) |
| C26—N4—N5—C14 | −108.8 (3) | N4—N5—C14—S2 | −171.95 (18) |
| N1—N2—C1—N3 | 5.9 (4) | N5—N4—C15—O3 | −3.5 (4) |
| N1—N2—C1—S1 | −177.69 (19) | C26—N4—C15—O3 | −176.0 (2) |
| N2—N1—C2—O1 | −8.2 (4) | N5—N4—C15—C16 | 176.8 (2) |
| C13—N1—C2—O1 | 176.4 (2) | C26—N4—C15—C16 | 4.3 (4) |
| N2—N1—C2—C3 | 173.1 (2) | O3—C15—C16—C17 | −0.5 (4) |
| C13—N1—C2—C3 | −2.4 (4) | N4—C15—C16—C17 | 179.2 (2) |
| O1—C2—C3—C4 | 5.2 (4) | O3—C15—C16—C25 | −178.7 (3) |
| N1—C2—C3—C4 | −176.2 (2) | N4—C15—C16—C25 | 1.0 (4) |
| O1—C2—C3—C12 | −173.6 (2) | C25—C16—C17—C18 | −0.3 (4) |
| N1—C2—C3—C12 | 5.1 (4) | C15—C16—C17—C18 | −178.6 (3) |
| C12—C3—C4—C5 | −0.3 (4) | C16—C17—C18—C19 | −1.1 (4) |
| C2—C3—C4—C5 | −179.0 (2) | C17—C18—C19—C20 | 1.5 (4) |
| C3—C4—C5—C6 | 0.3 (4) | C18—C19—C20—C21 | 179.2 (3) |
| C4—C5—C6—C7 | 0.1 (4) | C18—C19—C20—C25 | −0.5 (4) |
| C5—C6—C7—C8 | 178.8 (2) | C25—C20—C21—C22 | 0.8 (4) |
| C5—C6—C7—C12 | −0.5 (4) | C19—C20—C21—C22 | −178.9 (3) |
| C6—C7—C8—C9 | −179.7 (2) | C20—C21—C22—C23 | −1.8 (5) |
| C12—C7—C8—C9 | −0.4 (4) | C21—C22—C23—C24 | 1.0 (4) |
| C7—C8—C9—C10 | −1.2 (4) | C22—C23—C24—C25 | 0.8 (4) |
| C8—C9—C10—C11 | 1.4 (4) | C22—C23—C24—C26 | −177.7 (2) |
| C9—C10—C11—C12 | 0.1 (4) | C17—C16—C25—C24 | 179.7 (3) |
| C9—C10—C11—C13 | −177.2 (2) | C15—C16—C25—C24 | −2.1 (4) |
| C10—C11—C12—C3 | 179.0 (2) | C17—C16—C25—C20 | 1.4 (4) |
| C13—C11—C12—C3 | −3.8 (4) | C15—C16—C25—C20 | 179.6 (2) |
| C10—C11—C12—C7 | −1.7 (4) | C23—C24—C25—C16 | 179.9 (3) |
| C13—C11—C12—C7 | 175.5 (2) | C26—C24—C25—C16 | −1.6 (4) |
| C4—C3—C12—C11 | 179.1 (2) | C23—C24—C25—C20 | −1.8 (4) |
| C2—C3—C12—C11 | −2.1 (4) | C26—C24—C25—C20 | 176.7 (2) |
| C4—C3—C12—C7 | −0.2 (4) | C21—C20—C25—C16 | 179.4 (2) |
| C2—C3—C12—C7 | 178.6 (2) | C19—C20—C25—C16 | −0.9 (4) |
| C8—C7—C12—C11 | 1.9 (4) | C21—C20—C25—C24 | 1.0 (4) |
| C6—C7—C12—C11 | −178.8 (2) | C19—C20—C25—C24 | −179.3 (3) |
| C8—C7—C12—C3 | −178.8 (2) | N5—N4—C26—O4 | −1.8 (4) |
| C6—C7—C12—C3 | 0.5 (4) | C15—N4—C26—O4 | 170.8 (3) |
| N2—N1—C13—O2 | 0.2 (4) | N5—N4—C26—C24 | 179.8 (2) |
| C2—N1—C13—O2 | 175.7 (2) | C15—N4—C26—C24 | −7.7 (4) |
| N2—N1—C13—C11 | −178.7 (2) | C23—C24—C26—O4 | 6.3 (4) |
| C2—N1—C13—C11 | −3.2 (4) | C25—C24—C26—O4 | −172.3 (3) |
| C10—C11—C13—O2 | 4.8 (4) | C23—C24—C26—N4 | −175.4 (2) |
| C12—C11—C13—O2 | −172.6 (2) | C25—C24—C26—N4 | 6.1 (4) |
Hydrogen-bond geometry (Å, º)
| D—H···A | D—H | H···A | D···A | D—H···A |
| N2—H2···O1i | 0.88 | 2.35 | 3.074 (3) | 140 |
| N2—H2···O1ii | 0.88 | 2.33 | 2.993 (3) | 132 |
| N3—H31···O2iii | 0.88 | 2.09 | 2.912 (3) | 154 |
| N5—H5···O3iii | 0.88 | 2.55 | 3.301 (3) | 144 |
| N5—H5···O3iv | 0.88 | 2.52 | 2.988 (3) | 114 |
| N6—H61···O4i | 0.88 | 2.21 | 3.015 (3) | 152 |
Symmetry codes: (i) x−1, y, z; (ii) −x, −y+2, −z+1; (iii) x+1, y, z; (iv) −x+2, −y+1, −z.
Footnotes
Supplementary data and figures for this paper are available from the IUCr electronic archives (Reference: BT5920).
References
- Agilent (2012). CrysAlis PRO Agilent Technologies, Yarnton, England.
- Barbour, L. J. (2001). J. Supramol. Chem. 1, 189–191.
- Pessoa, C., Lotufo, L. V. C., de Moraes, M. O., Cavalcanti, S. M. T., Coêlho, L. C. D., Hernandes, M. Z., Leite, A. C. L., De Simone, C. A., Costa, V. M. A. & Souza, V. M. O. (2010). ChemMedChem, 5, 523–528. [DOI] [PubMed]
- Sheldrick, G. M. (2008). Acta Cryst. A64, 112–122. [DOI] [PubMed]
- Westrip, S. P. (2010). J. Appl. Cryst. 43, 920–925.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Crystal structure: contains datablock(s) global, I. DOI: 10.1107/S1600536812021812/bt5920sup1.cif
Structure factors: contains datablock(s) I. DOI: 10.1107/S1600536812021812/bt5920Isup2.hkl
Supplementary material file. DOI: 10.1107/S1600536812021812/bt5920Isup3.cml
Additional supplementary materials: crystallographic information; 3D view; checkCIF report