Abstract
Acyl homoserine lactone (AHL)-based quorum sensing commonly refers to cell density-dependent regulatory mechanisms found in bacteria. However, beyond bacteria, this cell-to-cell communication mechanism is poorly understood. Here we show that a methanogenic archaeon, Methanosaeta harundinacea 6Ac, encodes an active quorum sensing system that is used to regulate cell assembly and carbon metabolic flux. The methanogen 6Ac showed a cell density-dependent physiology transition, which was related to the AHL present in the spent culture and the filI gene-encoded AHL synthase. Through extensive chemical analyses, a new class of carboxylated AHLs synthesized by FilI protein was identified. These carboxylated AHLs facilitated the transition from a short cell to filamentous growth, with an altered carbon metabolic flux that favoured the conversion of acetate to methane and a reduced yield in cellular biomass. The transcriptomes of the filaments and the short cell forms differed with gene expression profiles consistent with the physiology. In the filaments, genes encoding the initial enzymes in the methanogenesis pathway were upregulated, whereas those for cellular carbon assimilation were downregulated. A luxI–luxR ortholog filI–filR was present in the genome of strain 6Ac. The carboxylated AHLs were also detected in other methanogen cultures and putative filI orthologs were identified in other methanogenic genomes as well. This discovery of AHL-based quorum sensing systems in methanogenic archaea implies that quorum sensing mechanisms are universal among prokaryotes.
Keywords: carboxylated acyl homoserine lactones, filI-encoded AHL synthase, methanogenic archaea, physiology transition, quorum sensing
Introduction
Quorum sensing is a well-characterized gene-regulatory mechanism that coordinates diverse social behaviours in bacteria, such as biofilm formation, antibiotic resistance, production of luminescence, exotoxins and exopolysaccharides, and uptake of extracellular DNA (Hastings and Greenberg, 1999; Waters and Bassler, 2005). In this process, bacteria communicate through secreted signal molecules or autoinducers, which are involved in gene regulation. The Gram-negative bacteria use acyl homoserine lactones (AHLs) as a quorum sensing signal to achieve regulation through the luxI–luxR homolog system, whereas Gram-positive bacteria use small peptides as autoinducers. Despite the ubiquity of quorum sensing systems in bacteria, quorum sensing has yet to be conclusively identified in archaea, except that a bacterial biosensor was reported to detect potential quorum sensing signals from a halophilic archaeon (Paggi et al., 2003) and genome analyses suggest that LuxS-based AI-2 signalling may be present (Sun et al., 2004).
We previously isolated an obligate aceticlastic methanogenic archaeon, Methanosaeta harundinacea 6Ac, from the up-flow anaerobic sludge bed granules in an anaerobic digester (Ma et al., 2006). Intriguingly, this methanogen shows a cell density-dependent cell assembly (as described below), making it a good model system for the study of quorum sensing behaviour in archaea. In the present study, a bacterial luxI–luxR-like circuit was observed in M. harundinacea 6Ac and a luxI homolog called the filI gene was shown to encode AHL synthase. This archaeal enzyme synthesized a group of carboxylated AHL with 10–14 carbon atoms in the acyl chain. Furthermore, these carboxyl-AHLs acted as the quorum sensing signal in regulating the physiology of M. harundinacea 6Ac. Therefore this methanogenic archaeon uses a quorum sensing system similar to that used by Gram-negative bacteria.
Materials and methods
Methanogen strain and growth conditions
M. harundinacea 6Ac was maintained in our laboratory and routinely cultured in a pre-reduced basal medium containing 50 mM sodium acetate, yeast extract (0.05%, w/v) and peptone (0.05%, w/v) as described previously (Doddema and Vogels, 1978; Ma et al., 2006). To promote the formation of filaments, the acetate in growing cultures was replenished up to three times with 50 mM sodium acetate.
N-acyl-homoserine lactone bioassay
Agrobacterium tumefaciens NTL4, which carries the plasmid pZLR4, was used as an N-acyl-homoserine lactone reporter. Plasmid pZLR4 contains a traG::lacZ fusion and traR (Steindler and Venturi, 2007). The plate assay for detection of AHLs was performed as described previously (Hwang et al., 1994). Briefly, AB agar containing 0.2–0.5% glucose and 40 μg ml−1 X-gal was poured into Petri dishes, then half of the plate was overlaid with 0.7% agar containing the reporter strain NTL4, whereas another half was overlaid with only 0.7% agar as a blank. After the overlay solidified, 5 μl of the test liquid was spotted onto to the soft agar on both halves of the plate. The plates were incubated at 28 °C for 12–48 h. Formation of blue spots indicated the presence of AHLs in the test sample. AHLs in the spent culture were quantified according to the method of Cha et al. (1998).
Cloning of filI gene, and expression and purification of FilI protein
The genomic DNA of M. harundinacea 6Ac was extracted as described previously (Zhou et al., 1996) and was used as template for PCR amplification of ORF00438 (GenBank no. HQ188282) (filI). The primer pair shown in Supplementary Table S3 was used for PCR amplification of the filI gene. PCR amplification was performed using Pfu DNA polymerase (Promega, Madison, WI, USA) for 30 cycles, with each cycle consisting of denaturation at 95 °C for 45 s, annealing at 59 °C for 1 min and elongation at 72 °C for 5 min. The PCR product was purified using the 3S Spin Agarose Gel DNA Purification kit (Shanghai Biocolor Bioscience and Technology Company, Shanghai, China) and cloned into the pET28a vector for expression in the Escherichia coli Rosseta (DE3) strain. The FilI protein was purified by HisTrap HP and Q ion exchange column chromatography (GE Healthcare, Piscataway, NJ, USA).
Enzymatic synthesis of AHLs by FilI
The principle of in vitro enzymatic synthesis of AHLs was described elsewhere (More et al., 1996; Val and Cronan, 1998). Briefly, S-adenylmethionine was the substrate for the homoserine lactone moiety and the cell-free extract of strain 6Ac was the source of acyl-carrier proteins (ACPs), according to the presence of a β-ketoacyl-acyl carrier protein synthase gene fabH (Mhar_2367) in the genome, although no ACP homologue is found in the genome. Cells in mid-log phase were collected by centrifugation, resuspended in TEDG buffer (10 mM Tris-HCl (pH 8.0), 0.1 mM EDTA, 0.1 mM dithiothreitol, 5% glycerol), and disrupted by ultrasonication for 30 min on ice. The cell lysate was centrifuged at 20 000 g for 1 h and the supernatant of about 5 mg protein per ml was used for ACPs. FilI synthesis reactions (200 μl) were performed in microfuge tubes at 37 °C for 30 min. The reaction mixture contained 10 mM Tris-HCl (pH 7.4), 330 mM NaCl, 15% glycerol, 0.7 mM dithiothreitol, 2 mM EDTA, 25 mM MgSO4, 0.1 mM FeSO4, and 0.15 mM S-adenylmethionine and 60 μl cell-free extract of strain 6Ac, unless otherwise indicated. The reaction was initiated by adding 6 μg per ml of His6-FilI and stopped by adding three volumes of ethyl acetate. The ethyl acetate phase was collected and dried in a rotary vacuum evaporator at 30 °C. The residue was re-suspended in methanol for purification of AHLs.
Purification of FilI-synthesized AHLs
AHLs synthesized by FilI were purified using an Agilent ZORBAX Extend-C18 Column (4.6 × 250 mm, 5 μm particle size) on Agilent's 1100 Series HPLC, and monitored with a Finnigan SpectraSYSTEM UV6000LP PDA Detector and LCQ Deca XPplus ion-trap mass spectrometer (Thermo-Finnigan, San Jose, CA, USA) (Ortori et al., 2007). The purification parameters were as follows: column temperature 15 °C; mobile phase-A, water with 0.01% trifluoroacetic acid; mobile phase-B, acetonitrile with 0.01% frifluoroacetic acid; flow rate, 0.8 ml min−1; gradient profile, linear increase of phase-B from 30 to 90% over 5–65 min. The column was re-equilibrated for a total of 15 min prior to 50 μl loading. The three AHL compounds were collected in the elute fractions with a retention time of 25–35 min.
Mass spectral determination of AHLs
Electrospray ionization mass spectra were recorded in the positive ionization mode using a LCQ Deca XPplus ion-trap mass spectrometer (Thermo-Finnigan) (Cataldi et al., 2009). Samples in 50% methanol were infused directly into the source at 5 μl min−1 using a syringe pump. The transfer capillary temperature and spray voltage were set at 275 °C and 5.5 kV, respectively (Sharif et al., 2008). The sheath gas flow rate was set to 12 arbitrary units and the tube lens offset was set to 25 V. For MSn analysis, selected precursor ions were isolated with a width of 3 m/z, and the collision energy was optimized to obtain the stable and entire product ion spectra. Based on above mass spectrometric conditions, the specific fragments of the AHL standards (N-capryloyl-DL-homoserine lactone, N-caproyl-DL-homoserine lactone, N-lauroyl-DL-homoserine lactone, N-(β-ketooctanoyl)-L-homoserine, N-heptanoyl-DL-homoserine lactone and N-(β-ketocaproyl)-DL-homoserine lactone, N-tetradecanoyl-DL-homoserine lactone and N-decanoyl-DL-homoserine lactone; Sigma-Aldrich, St Louis, MO, USA) were further characterized. The core homoserine lactone (HSL) moiety was monitored at m/z 102.05 and electrospray ionization mass spectrometry (ESI-MS)/MS spectra were optimized over the range m/z 50–110. Using ESI (positive ion mode) coupled with Fourier transform ion cyclotron resonance MS APEX IV (ESI-FT-ICR; Bruker Daltonics, Billerica, MA, USA), high-resolution data of the compounds in methanol were determined. The Bruker Compass Data Analysis software (version 4.0) was used for data acquisition and processing (Cataldi et al., 2008).
NMR spectroscopic analysis
NMR spectra were acquired with a Varian Mercury 600 MHz NMR spectrometer (Varian Corp., Palo Alto, CA, USA) operating at 600.13 MHz proton frequency by using a 3 mm inverse geometry broadband probe head equipped with an actively shielded z-gradient coil (907(1H) 7.3/9.8 ms CDCl3/0.01 M sodium carbonate) (Pearson et al., 1995; Frommberger et al., 2005). 1H NMR spectra (AQ, 5.23 s; relaxation delay, 0.1 s; exponential line-broadening, 0.3 Hz) were recorded with 20–907 pulses.
Cell protein concentration determination
Protein concentrations were determined using the BCA Protein Assay kit (Thermo Scientific, Rockford, IL, USA), using bovine serum albumin as the calibration standard.
qPCR experiments
Quantitative PCRs (qPCRs) were performed in eight strip PCR tubes (Axygen, Union City, CA, USA), and the reaction signals were generated by the binding of SYBR green to double-stranded DNA. All qPCR experiments were performed using an ABI Prism 7000 Sequence Detection System (Applied Biosystems, Foster City, CA, USA). The primers used for quantitative real-time PCR analysis are listed in Supplementary Table S3 and were designed using the Premier express 2.0 software, Primer Premier 5.0 and Oligo 6.0. Two pairs of primers, 16SRTF/16SRTR and filiRTF/filiRTR, amplified the 16S rRNA and the ORF00438 gene of strain 6Ac, respectively. The specificity of the primer sets was confirmed by sequencing the amplicons (215 bp). Plasmids bearing the 16S rRNA (AY970347) or the filI gene of strain 6Ac were used as standards. Plasmids were extracted by the Tianprep Mini Plasmid Kit (Tiangen Biotech, Beijing, China) and purified using the 3S Spin Agarose Gel DNA Purification kit (Shanghai Biocolor Bioscience and Technology Company). The DNA preparations were quantified by using a NanoDrop ND-1000 UV-Vis Spectrophotometer (Thermo Scientific, Wilmington, DE, USA). The 16S rRNA copy number was calculated as described previously (Yu et al., 2005).
qPCR experiments were performed using the 16SRTF/16SRTR and filiRTF/filiRTR primer sets (Supplementary Table S3). Each qPCR mixture contained 12.5 μl of 2 × SYBR green mastermix (Applied Biosystems), 1 μl of DNA template, 100 nM of each primer and ddH2O to a final volume of 25 μl. The PCR was initiated at 50 °C for 2 min to optimize AmpErase uracil-N-glycosylase activity, followed by denaturation at 95 °C for 10 min and 40 cycles of amplification as follows: DNA denaturation at 95 °C for 30 s, primer annealing at 57 °C for 40 s and elongation at 72 °C for 40 s. Fluorescence data were collected during the elongation steps. The reactions were all performed in three replicates.
Microarray procedure
For transcriptome studies, M. harundinacea 6Ac was grown in 30 mM sodium acetate to prevent conversion of short cells into filaments, and growth of cultures was monitored by determining methane production. Purified N-carboxyl-C12-HSL (m/z 346) (final concentration, 50 nM) was added to triplicate cultures during the mid-log growth phase. After further incubating until the late-logarithmic phase, the formation of filaments was verified before extracting total RNAs with the TRIZol reagent (Invitrogen, Carlsbad, CA, USA) and further purification using the RNeasy Mini kit (Qiagen, Hilden, Germany). The Agilent Low Input Quick Amp Labeling kit (Agilent Technologies, Santa Clara, CA, USA) was used to synthesize cDNA from the total RNA samples and subsequently produce the amino allyl-modified cDNA according to the manufacturer's instructions. The amino allyl-labelled cDNA was then linked to Cy3 NHS ester (GE Healthcare). The fluorescently labelled cDNA was hybridized with an Agilent M. harundinacea 6Ac Custom 8 × 15 K Microarray according to the manufacturer's protocol. The Feature Extraction Software (Agilent Technologies) was used for data acquisition and GeneSpring (Agilent Technologies) was used for further data processing according to published procedures (Gobert et al., 2009). To validate the microarray data, qPCRs were performed using the primer pairs listed in Supplementary Table S3. The PCR mixtures included 200 nM primers, 1–100 ng of cDNA and 10 μl of 2 × SYBR Green PreMix (TaKaRa, Dalian, China). The parameters were 95 °C for 30 s, followed by 40 cycles of 95 °C for 5 s, 60 °C for 15 s and 72 °C for 31 s.
Results
Cell density-dependent morphology change of M. harundinacea
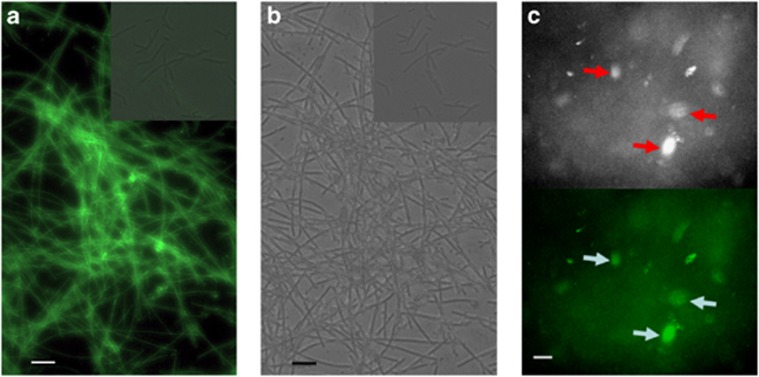
During growth in the up-flow anaerobic sludge bed granules, M. harundinacea 6Ac formed rigid, mane-like filaments encasing multiple cells in a common shell. However, upon sub-culturing these cells in 50 mM acetate, the optimal concentration for growth, the cell assemblages became substantially shorter with less than three linked cells. As cell density increased upon supplementing the spent cultures with additional substrate, rigid mane-like filaments (Figures 1a and b) re-formed. Interestingly, the shorter ones (inserts in Figures 1a and b) could become filaments if they were concentrated by centrifugation, or resuspended in the conditioned media from the spent filament cultures. Furthermore, the filament cells emitted intensive fluorescence when excited at 420 nm, owing to oxidation of the methanogen-specific cofactor F420, whereas this ability was abrogated in the short cell morphology (Figure 1a). Similarly, colony formation was promoted on agar plates containing conditioned medium (Figure 1c), whereas colonies were rarely observed on plates containing only the basic medium. These observations suggest that M. harundinacea used extracellular signals to control cell assembly in a cell density-dependent manner, which resembles quorum sensing behaviours reported in bacteria.
Figure 1.
Induction of filaments and colony formation by the filamentous spent culture of M. harundinacea. (a) A microscope image of the filaments in conditioned medium with spent culture of filamentous cells showing fluorescence at 420 nm, whereas the short cells (insert) show no fluorescence. Bar, 5 μm. (b) Phase-contrast microscopic image of the filaments in conditioned medium with spent culture of filamentous cells and short cells (insert). Bar, 5 μm. (c) Microscope images of colonies forming on conditioned agar medium with spent culture of filamentous cells. The arrows indicate the presence of colonies using a light microscope (upper) and illumination at 420 nm (lower). Bar, 1 mm.
Identification of the possible quorum sensing signals in M. harundinacea
To determine the identity of the extracellular signal produced by M. harundinacea, medium from cell-free spent cultures of filamentous cells was extracted with ethyl acetate and the ethyl acetate extract was tested for various bioactive molecules. Interestingly, the 300–1500 times concentrated solutions derived from the ethyl acetate extracted spent media triggered the expression of an AHL reporter strain, Agrobacterium sp. NTL4, suggesting the possible presence of AHL-like molecules. By using β-ketooctanoyl-L-homoserine lactone as the standard, AHL(s) at concentrations of 100–500 nM and 10–27 μM were determined in the short cell and filament cultures, respectively. Next, the ethyl acetate extract was examined by tandem ESI-MS and compounds with a molecular ion m/z of 318.2, 274.2, 284.2, 346.2, 374.2 and 302.3 were observed in a relatively high abundance (Supplementary Figure S1a). These compounds all produced a diagnostic HSL ion m/z 102 in their tandem ESI-MS (Supplementary Figure S1a), similar to the chemical β-ketooctanoyl-L-homoserine lactone (Supplementary Figure S1b). This result confirmed the presence of AHLs in the sample. However, the AHL level in the medium was too low to be purified for further structural identification.
Determination of an AHL synthase gene, filI, in M. harundinacea
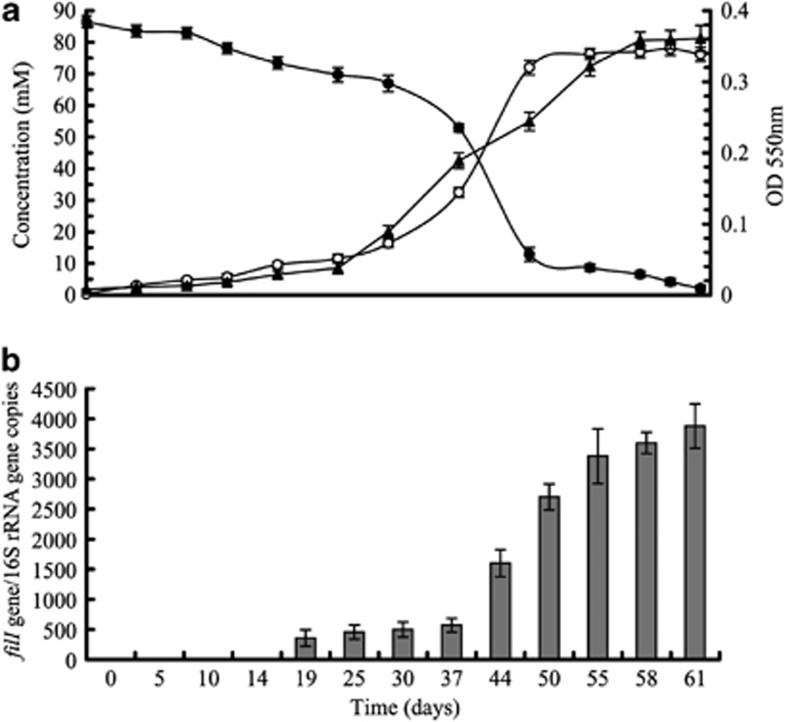
By analogy with the bacterial systems, LuxI would be the key enzyme for this process. Thus, we used the well-defined bacterial luxI orthologues (Supplementary Figure S2) as probes to query the completed genome sequence of M. harundinacea using TBLASTN. The best match was ORF00438 (HQ188282), which is annotated as a ‘multi-sensor signal transduction histidine kinase', and the CHASE 4 domain of the protein shows 39.6% identity to AhlI, the LuxI autoinducer synthase of Erwinia chrysanthemi (GenBank accession number AAM46699). Based on this result, ORF00438 was renamed as filI to reflect its role in inducing the filamentous morphology. The transcript level of filI was examined during growth and it increased with cell density, which is typical of many bacterial quorum sensing-regulated genes (Figure 2).
Figure 2.
filI expression during growth. (a) Culture absorption (open circles) and methane formation (triangles) by M. harundinacea 6Ac during growth on acetate (closed circles). (b) Copies of filI mRNA per 16S rRNA gene estimated by qPCR using a pair of filI-specific primers listed in Supplementary Table S3. Copy numbers were calculated from duplicate cultures. The error bars show standard deviation.
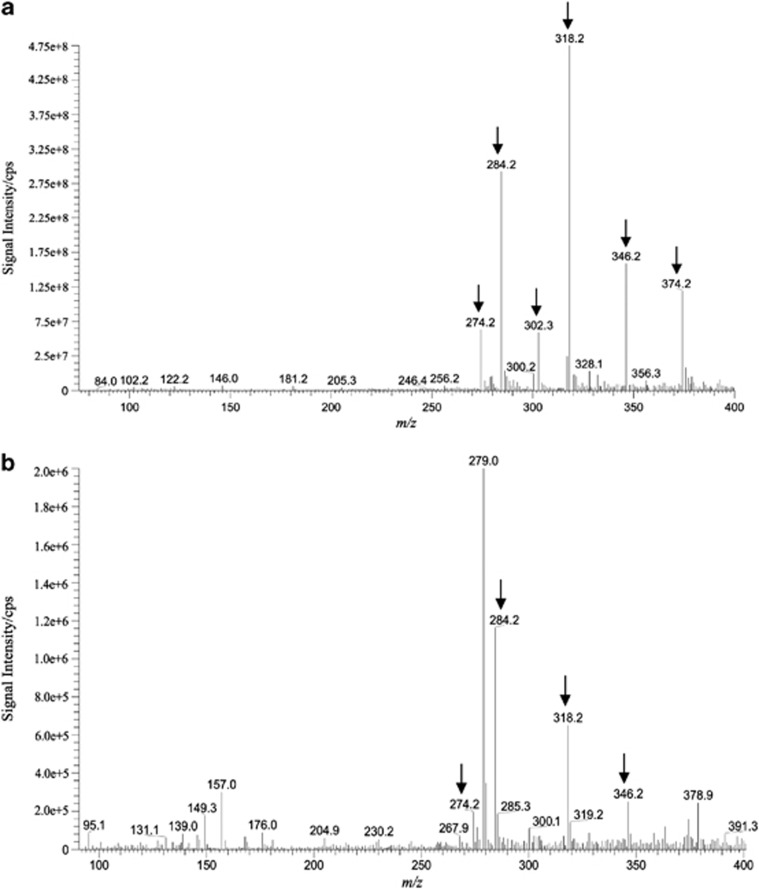
To obtain more definitive evidence for the biochemical function of filI, it was overexpressed in E. coli, and the His6-tagged FilI protein was purified to examine its enzymatic function. According to the previously published protocols for Agrobacterium (More et al., 1996), S-adenylmethionine, cell-free extract of M. harundinacea 6Ac and the purified FilI protein were combined for an in vitro AHL synthesis assay. Six major products were detected by ESI-MS analysis with MS peaks at m/z 318.2, 284.2, 346.2, 374.2, 274.2 and 302.3 (Figure 3a). The ion intensities were substantially increased compared with the reaction mixtures without FilI (Figure 3b); additionally, the six compounds all generated the characteristic HSL ion m/z 102 in the tandem ESI-MS (Figure 4, and Supplementary Figures S3 and S4). Therefore FilI was determined to be a methanogenic AHL synthase.
Figure 3.
ESI-MS profile of the FilI synthetic mixture. (a) Each of the six compounds indicated by arrows was produced by FilI and formed the characteristic daughter ion m/z 102 shown in Figure 4, and Supplementary Figures S3 and S4. (b) The cell-free extract of M. harundinacea without FilI.
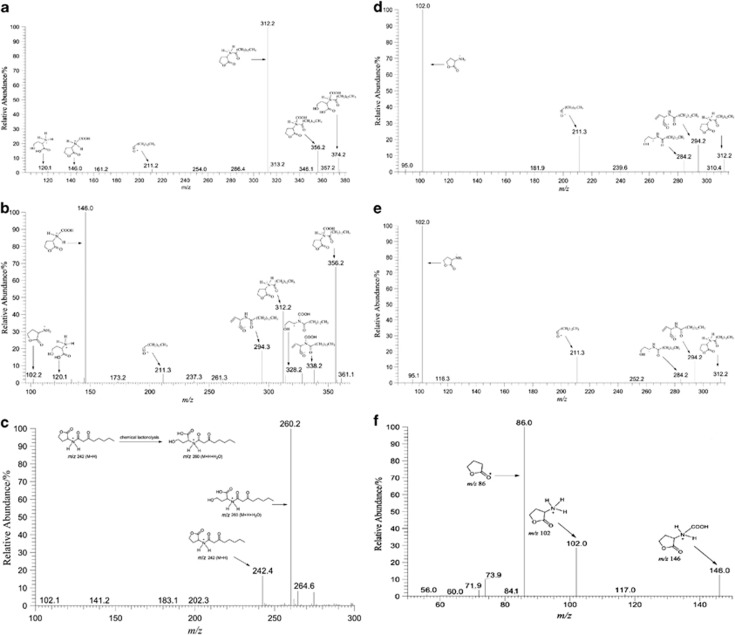
Figure 4.
MS/MS daughter scans of Compound-1 (m/z 374) synthesized by FilI and the reference compound. (a) Compound-1 with open HSL ring. (b) Compound-1 with closed HSL ring. (c) N-β-ketooctanoyl-HSL (m/z 242) with open HSL ring using ESI-MS analysis. (d) Product ion (m/z 312) produced by Compound-1. (e) N-tetradecanoyl-DL-homoserine lactone (m/z 312). (f) Product ion (m/z 146) produced by Compound-1 showing an ion at m/z 102.
Structure elucidation of the FilI synthetic AHLs
To determine the chemical structures of the FilI-synthesized AHLs, these compounds were purified by high-performance liquid chromatography (HPLC) for tandem ESI-MS analysis. The three compounds (1: m/z 374.2; 2: m/z 346.2; and 3: m/z 318.2) synthesized by FilI all generated a fragment ion of m/z 102, the AHL characteristic ion product. Meanwhile a concomitant fragment ion of m/z 146 frequently occurred in the tandem ESI-MS (Figure 4, and Supplementary Figures S3 and S4), which produced a fragment ion of m/z 102 in its secondary ESI-MS spectrum, implying a carboxyl group connected to the HSL moiety in the AHLs. Following high-resolution ESI-MS, the molecular formula of the three compounds were determined to be C19H33NO5 (Compound-1, m/z 356.24319 [M+H] and m/z 374.25403 [M+H+H2O]), C17H29NO5 (Compound-2, m/z 328.21217 [M+H] and m/z 346.22273 [M+H+H2O]) and C15H25NO5 (Compound-3, m/z 300.18159 [M+H] and m/z 318.19126 [M+H+H2O]), respectively. The NMR spectrum of Compound-1 (Supplementary Figure S5) also possessed the characteristic resonance signals of AHL, but not the signal of the carboxyl group. To test the possible carboxyl group in Compound-1 it was treated with a vacuum drying process that mimicked the NMR preparation. Following a low-resolution ESI-MS analysis, the molecular ion m/z 374 [M+H+H2O] was remarkably decreased, whereas the abundance of m/z 312 [M+H] was significantly increased. Gas chromatography mass spectrometry experiment indeed detected CO2 during the process of vacuum drying for Compound-1 (m/z 374), thus determining that the undetected carboxylate group by 1H NMR was probably attributed to decarboxylation of Compound-1 during preparation for NMR.
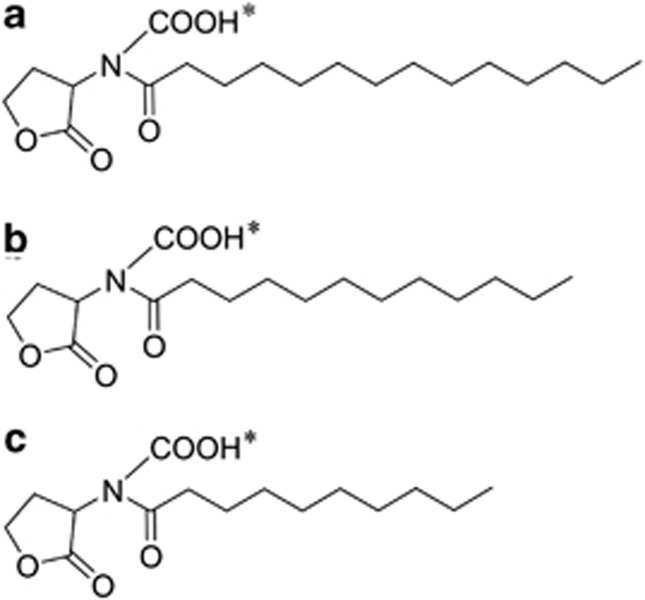
Based on the tandem ESI-MS data, two possible linkages of the carboxyl group, either connected with the NH group or the lactone ring, were predicted in Compound-1. To identify the carboxyl modification site, Compound-1 was further subjected to deuterium substitution by dissolved in CD3OD at a volume ratio of 1:1 at −20 °C for 3 days. This experiment is based on the following assumptions: (1) the exchangeable hydrogen in the carboxyl group can be readily substituted by a deuterium; (2) when the carboxyl group connects with the NH, 4 and 2 exchangeable hydrogens would be present in the HSL-opened and HSL-closed Compound-1 as shown in Supplementary Figure S6a, respectively; (3) when the carboxyl group connects with the lactone ring, an extra exchangeable hydrogen would be present (5 in the HSL-opened and 3 in the HSL-closed Compound 1, respectively). Undoubtedly, Supplementary Figure S6b showed 4 and 2 deuterium substitutions, but not 5 and 3 in the CD3OD-treated, HSL-opened (m/z 374) and HSL-closed (m/z 356) Compound-1, thus confirming the linkage of the carboxyl group with the amino group. Therefore, Compound-1 (m/z 374) and its two homologues (Compound-2: m/z 346 and Compound-3: m/z 318) were determined to be N-carboxyl-C14-HSL, N-carboxyl-C12-HSL and N-carboxyl-C10-HSL (Figure 5), respectively. The three compounds were detected in the spent media as well, indicating that FilI is the synthase for the AHLs produced by M. harundinacea.
Figure 5.
Chemical structures of the FilI synthetic carboxyl-AHLs. (a) Compound-1: N-carboxyl-C14-HSL. (b) Compound-2: N-carboxyl-C12-HSL. (c) Compound-3: N-carboxyl-C10-HSL. The asterisk refers to the exchangeable hydrogen in each compound.
Carboxyl-AHLs act as quorum sensing signals in regulating cell form and physiology
To determine whether the FilI-synthesized carboxyl-AHLs were indeed the signal molecules responsible for the cell density-dependent morphological and physiological changes described above, each of the three purified compounds was added to liquid cultures of M. harundinacea at their late log phase or mixed in agar medium at a final concentration of 6–10 nM. With these AHLs, a higher ratio of filamentous cells in the liquid culture after 2–3 weeks and colonies on the agar plates after about 4 weeks were found. By contrast, the cultures supplemented with m/z 256 and m/z 284, the AHLs present in Gram-negative bacteria, isolated in the FilI synthetic mixture, remained almost exclusively in the short cell phenotype and formed very few colonies on plates (Table 1). In addition, a correlation was observed between the filament ratio and the amount of carboxyl-AHLs added (Supplementary Figure S7). These data support the hypothesis that the FilI-synthesized AHLs are likely the signal molecules to mediate cell morphology change of M. harundinacea.
Table 1. Effect of FilI synthetic carboxyl-AHLs on the formation of colonies and filamentous cells of M. harundiacea.
| Supplied AHLs | ESI-MS ions (m/z)a | Colonies per serum bottleb | Filamentous cells ratio (%)b |
|---|---|---|---|
| None | 2.5±1.3 | 0 | |
| N-carboxyl-decanoyl-HSL | 318.2/300.2 | 12.8±5.7 | >80 |
| N-carboxyl-lauroyl-HSL | 346.2/328.2 | 5.5±2.1 | >50 |
| N-carboxyl-tetradecanoyl-HSL | 374.2/356.2 | 10.4±3.8 | >90 |
| N-decanoyl-DL-HSL | 256.3 | 2.7±1.5 | 0 |
| N-lauroyl-DL-HSL | 284.2 | 7.1±3.6 | 0 |
Abbreviations: AHL, acyl homoserine lactone; ESI-MS, electrospray ionization mass spectrometry.
Ion structure with an open/closed lactone ring.
Data from three replicates.
Given the obvious differences in cellular morphology between the short cells and filaments, the two forms were expected to show differences in their metabolism and physiology. During growth on acetate as the sole carbon source as described under Materials and methods, the filaments yielded 31% more methane, with a concomitant decrease in cellular protein production (38.5% less), than short cells. Thus the carbon flux in the filamentous cells favoured methane production. Similarly, acetate conversion by filaments possessed a lower apparent Ks value (0.42±0.038 mM) and higher reaction rate (Vmax, 0.64±0.015 mM·h−1·mg−1 cell protein) compared with the short cells (Ks: 0.62±0.022 mM; Vmax: 0.50±0.028 mM·h−1·mg−1), further indicating the distinct carbon metabolic patterns between the two morphotypes.
To understand how quorum sensing might influence carbon metabolism regulation in M. harundinacea, gene expression profiles between the short cells and filaments induced by N-carboxyl-C12-HSL (m/z 346) were compared. The transcriptomes of the two cell types in the late log phase showed that the majority of the genes involved in aceticlastic methanogenesis were downregulated in the filaments (Supplementary Table S1), such as the CO dehydrogenase/acetyl-CoA synthase operon and the electron transfer chain F420 dehydrogenase operon. This result contrasted with the greater conversion of acetate to CH4 in the filaments. However, two acetyl-CoA synthetase genes (ORF 00570 and 00732), the key components for the initial step of the aceticlastic methanogenesis pathway, were both upregulated in the filaments. Genes involved in cellular biosynthetic reactions (encoding pyruvate:ferredoxin oxidoreductase and phosphoenol-pyruvate synthase), transcription (RNA polymerase) and translation (elongation factors) were also downregulated in the filaments, which was consistent with their lower biomass yield. The genes for a number of F420-dependent enzymes were all highly expressed in the filaments, possibly a cause for the increased auto-fluorescence. Although, an outer-membrane protein and a filament induction protein were highly expressed in the filaments, these were likely consequences of the specific increase in the production of structural proteins found in the filament shell. As expected, qPCR confirmed the differential expression of each of these genes in the two cell types (Supplementary Table S1).
Discussion
The AHL-based quorum sensing regulation had previously only been described in Bacteria. In this study, a methanogenic archaeon M. harundinacea was shown to use modified AHL molecules, carboxyl-AHLs, to regulate a density-dependent cell behaviour and carbon flux. In order to rule out the possibility that the detected AHLs might have resulted from bacterial contamination, as methanogens frequently live in consortia with syntrophic bacteria for conversion of fatty acids to methane, extensive studies confirmed the purity of the culture. No growth was observed following extensive incubation with rich media in the absence of a substrate for methanogens as well as in the presence of high concentrations of the archaea-specific antibiotics. In addition, PCR amplification of bacterial 16S rRNA genes repeated negative results.
Like many bacterial AHL producers, M. harundinacea possesses a luxI–luxR orthologue, called filI–filR, in the genome. While the putative FilR possesses a homologue to the bacterial LuxR family on the basis of the receiver domains, it groups with the other types of regulators in other methanogen genomes and forms a distinct clade at 23.5% amino-acid sequence identity with bacterial LuxRs. This suggests a divergent evolution of methanogenic FilR and bacterial LuxRs.
Similar to Gram-negative bacteria, M. harundinacea produces AHLs with a luxI orthologue, filI. Unlike the bacterial AHLs, the three FilI-synthesized-AHLs all possess an extra carboxyl moiety on the N atom of the HSL ring. Thus, the methanogenic archaeon uses modified bacterial AHLs as the quorum sensing signals.
A similar AHL with a m/z 318 was also detected in cultures of Methanosarcina mazei and Methanothermobacter thermautotrophicus. In addition, using the filI gene as a probe and a similarity threshold of 0.01e, several signal transduction histidine kinase-encoding genes were identified in the genome sequences of other methanogens showing cell assembly phenotypes (Supplementary Table S2), such as M. mazei (Robinson, 1986), Methanosaeta concilii, Methanosaeta thermophila (Ahring et al., 1991) and Methanospirillum hungatei. These organisms undergo morphological changes or aggregation in a density-dependent manner (Xun et al., 1988). This suggests that AHLs may be the primary quorum sensing molecules used by methanogenic archaea. In addition, bacterial ACP gene homologies are found in some methanogens, such as M. hungatei and Candidatus Methanoregula boonei (Supplementary Table S2), although the concurrence of the ACP with filI gene homologies is not observed in methanogenic genomes. Methanogens may gain bacterial genes that are beneficial for environmental adaptation during evolution. Thus quorum sensing appears to be a universal regulation mechanism in prokaryote.
Finally, Methanosaeta species function as the crucial components of anaerobic digesters, not only for their ability to implement aceticlastic methanogenesis (Jetten et al., 1992), but also for their ability to form filaments that promote the formation of up-flow anaerobic sludge bed granules by serving as a scaffold for attachment by other organisms (Li et al., 2008). Therefore, the methanogenic AHLs might be useful for improving sludge granulation; much like that bacterial AHLs are used to promote phenol degradation in bioreactors (Valle et al., 2004).
Acknowledgments
We thank R Thauer for valuable discussions on the science impact of the study; Y Che for suggestion on chemical structure determination; and J Merritt for thoroughly editing the manuscript and G Xia for assistance with fluorescence imaging. This work was supported by National Natural Science Foundation of China under numbers 30621005, 30830007 and 31000011, and the Innovation Project of the PUMC Youth Foundation.
Footnotes
Supplementary Information accompanies the paper on The ISME Journal website (http://www.nature.com/ismej)
Supplementary Material
References
- Ahring BK, Alatriste-Mondragon F, Westermann P, Mah RA. Effects of cations on Methanosarcina thermophila TM-1 growing on moderate concentrations of acetate: production of single cells. Appl Microbiol Biotechnol. 1991;35:686–689. [Google Scholar]
- Cataldi TR, Bianco G, Abate S. Profiling of N-acyl-homoserine lactones by liquid chromatography coupled with electrospray ionization and a hybrid quadrupole linear ion-trap and Fourier-transform ion-cyclotron-resonance mass spectrometry (LC-ESI-LTQ-FTICR-MS) J Mass Spectrom. 2008;43:82–96. doi: 10.1002/jms.1275. [DOI] [PubMed] [Google Scholar]
- Cataldi TR, Bianco G, Abate S. Accurate mass analysis of N-acyl-homoserine-lactones and cognate lactone-opened compounds in bacterial isolates of Pseudomonas aeruginosa PAO1 by LC-ESI-LTQ-FTICR-MS. J Mass Spectrom. 2009;44:182–192. doi: 10.1002/jms.1479. [DOI] [PubMed] [Google Scholar]
- Cha C, Gao P, Chen YC, Shaw PD, Farrand SK. Production of acyl-homoserine lactone quorum-sensing signals by Gram-negative plant-associated bacteria. Mol Plant Microbe Interact. 1998;11:1119–1129. doi: 10.1094/MPMI.1998.11.11.1119. [DOI] [PubMed] [Google Scholar]
- Doddema HJ, Vogels GD. Improved identification of methanogenic bacteria by fluorescence microscopy. Appl Environ Microbiol. 1978;36:752–754. doi: 10.1128/aem.36.5.752-754.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frommberger M, Hertkorn N, Englmann M, Jakoby S, Hartmann A, Kettrup A, et al. Analysis of N-acyl homoserine lactones after alkaline hydrolysis and anion-exchange solid-phase extraction by capillary zone electrophoresis-mass spectrometry. Electrophoresis. 2005;26:1523–1532. doi: 10.1002/elps.200410365. [DOI] [PubMed] [Google Scholar]
- Gobert GN, Moertel LP, Brindley J, McManus DP. Developmental gene expression profiles of the human pathogen Schistosoma japonicum. BMC Genomics. 2009;10:128–146. doi: 10.1186/1471-2164-10-128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hastings JW, Greenberg EP. Quorum sensing: the explanation of a curious phenomenon reveals a common characteristic of bacteria. J Bacteriol. 1999;181:2667–2668. doi: 10.1128/jb.181.9.2667-2668.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hwang I, Li PL, Zhang L, Piper KR, Cook DM, Tate ME, et al. TraI, a LuxI homologue, is responsible for production of conjugation factor, the Ti plasmid N-acyl homoserine lactone autoinducer. Proc Natl Acad Sci USA. 1994;91:4639–4643. doi: 10.1073/pnas.91.11.4639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jetten M, Stams A, Zehnder A. Methanogenesis from acetate: a comparison of the acetate metabolism in Methanothrix soehngenii and Methanosarcina spp. FEMS Microbiol Let. 1992;88:181–197. [Google Scholar]
- Li J, Hu B, Zheng P, Qaisar M, Mei L. Filamentous granular sludge bulking in a laboratory scale UASB reactor. Biores Technol. 2008;99:3431–3438. doi: 10.1016/j.biortech.2007.08.005. [DOI] [PubMed] [Google Scholar]
- Ma K, Liu X, Dong X. Methanosaeta harundinacea, sp. nov., a novel acetate-scavenging methanogen isolated from a UASB reactor. Int J Sys Evol Microbiol. 2006;56:127–131. doi: 10.1099/ijs.0.63887-0. [DOI] [PubMed] [Google Scholar]
- More MI, Finger D, Stryker JL, Fuqua C, Eberhard A, Winans SC. Enzymatic synthesis of a quorum-sensing autoinducer using defined substrates. Science. 1996;272:1655–1658. doi: 10.1126/science.272.5268.1655. [DOI] [PubMed] [Google Scholar]
- Ortori CA, Atkinson S, Chhabra SR, Cámara M, Williams P, Barrett DA. Comprehensive profiling of N-acylhomoserine lactones produced by Yersinia pseudotuberculosis using liquid chromatography coupled to hybrid quadrupole–linear ion trap mass spectrometry. Anal Bioanal Chem. 2007;387:497–511. doi: 10.1007/s00216-006-0710-0. [DOI] [PubMed] [Google Scholar]
- Paggi RA, Martone CB, Fuqua C, De Castro RE. Detection of quorum sensing signals in the haloalkaliphilic archaeon Natronococcus occultus. FEMS Microbiol Let. 2003;221:49–52. doi: 10.1016/S0378-1097(03)00174-5. [DOI] [PubMed] [Google Scholar]
- Pearson JP, Passadori L, Iglewskit BH, Greenberg EP. A second N-acylhomoserine lactone signal produced by Pseudomonas aeruginosa. Proc Natl Acad Sci USA. 1995;92:1490–1494. doi: 10.1073/pnas.92.5.1490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson RW. Life cycles in the methanogenic archaebacterium Methanosarcina mazei. Appl Environ Microbiol. 1986;52:17–27. doi: 10.1128/aem.52.1.17-27.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharif DI, Gallon J, Smith CJ, Dudley E. Quorum sensing in Cyanobacteria: N-octanoyl-homoserine lactone release and response, by the epilithic colonial cyanobacterium Gloeothece PCC6909. ISME J. 2008;2:1171–1182. doi: 10.1038/ismej.2008.68. [DOI] [PubMed] [Google Scholar]
- Steindler L, Venturi V. Detection of quorum-sensing N-acyl homoserine lactone signal molecules by bacterial biosensors. FEMS Microbiol Lett. 2007;266:1–9. doi: 10.1111/j.1574-6968.2006.00501.x. [DOI] [PubMed] [Google Scholar]
- Sun J, Daniel R, Wagner-Döbler I, Zeng AP. Is autoinducer-2 a universal signal for interspecies communication: a comparative genomic and phylogenetic analysis of the synthesis and signal transduction pathways. BMC Evol Biol. 2004;4:36–46. doi: 10.1186/1471-2148-4-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valle A, Bailey MJ, Whiteley AS, Manefield M. N-acyl-L-homoserine lactones (AHLs) affect microbial community composition and function in activated sludge. Environ Microbiol. 2004;6:424–433. doi: 10.1111/j.1462-2920.2004.00581.x. [DOI] [PubMed] [Google Scholar]
- Val DL, Cronan JE., Jr In vivo evidence that S-adenosylmethionine and fatty acid synthesis intermediates are the substrates for the luxI family of autoinducer synthases. J Bacteriol. 1998;180:2644–2651. doi: 10.1128/jb.180.10.2644-2651.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waters CM, Bassler BL. Quorum sensing: cell-to-cell communication in bacteria. Annu Rev Dev Biol. 2005;21:319–346. doi: 10.1146/annurev.cellbio.21.012704.131001. [DOI] [PubMed] [Google Scholar]
- Xun LY, Boone DR, Mah RA. Control of the life cycle of Methanosarcina mazei S-6 by manipulation of growth conditions. Appl Environ Microbiol. 1988;54:2064–2068. doi: 10.1128/aem.54.8.2064-2068.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu Y, Lee C, Kim J, Hwang S. Group-specific primer and probe sets to detect methanogenic communities using quantitative real-time polymerase chain reaction. Biotechnol Bioeng. 2005;89:670–679. doi: 10.1002/bit.20347. [DOI] [PubMed] [Google Scholar]
- Zhou J, Bruns MA, Tiedje JM. DNA recovery from soils of diverse composition. Appl Environ Microbiol. 1996;62:316–322. doi: 10.1128/aem.62.2.316-322.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.