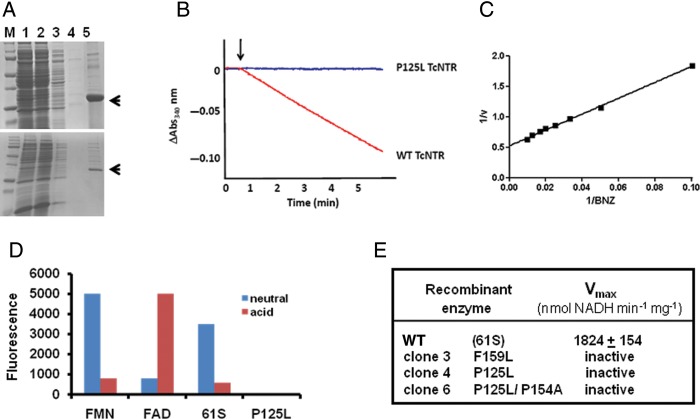
Figure 3.
Biochemical analysis of TcNTR from benznidazole (BNZ)-sensitive and -resistant Trypanosoma cruzi. A, Purification of recombinant TcNTR. Upper image, wild-type (WT; 61S) enzyme; lower, clone 4. Protein expression was induced by isopropyl-β-D-thiogalactopyranoside (Materials and Methods), and a clarified fraction (lane 1) was loaded onto a nickel–nitriloacetic acid column and the flow-through collected (lane 2). The column was washed with 50 mM and then 100 mM imidazole (lanes 3 and 4) and the recombinant protein eluted with 500 mM imidazole, 1% triton X-100 (lane 5). The 32-kDa TcNTR band is highlighted by an arrow. Recombinant protein from each of the resistant clones was purified in a similar manner. B, TcNTR activity was monitored (on the basis of absorbance [Abs] at a wavelength of 340 nm) by following oxidation of nicotinamide adenine dinucleotide, reduced (NADH; 100 μM), in the presence of WT or mutant (P125L, clone 4) enzyme (0.2 μg) and BNZ (100 μM). C, Activity (v) of the WT enzyme (nmol NADH per min per mg) was established by this assay with a fixed concentration of NADH (100 μM) in the presence of different levels of BNZ (10–100 μM). D, Fluorescence (excitation λ = 450 nm; emission λ = 535 nm) of the TcNTR cofactor (WT and P125L) and flavin mononucleotide (FMN) and flavin adenine dinucleotide (FAD) controls (in arbitrary units) under acidic and neutral conditions (Materials and Methods). E, Activity of WT and mutant TcNTRs.