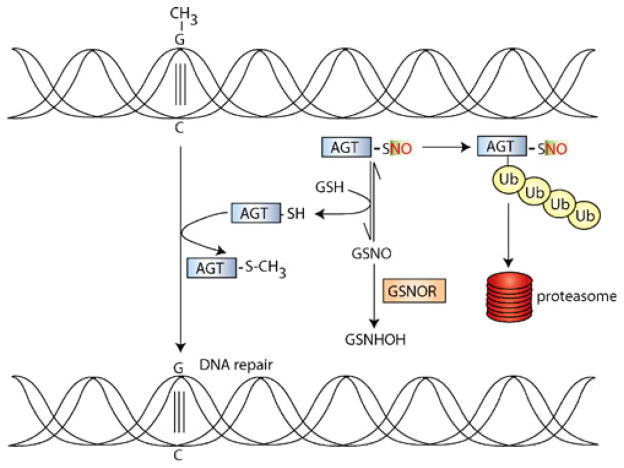
Fig. 3.
Deficiency of cellular GSNOR activity in the etiology of hepatic carcinoma. The O6-alkylguanine-DNA alkyl transferase (AGT) protein transfers the alkyl group (shown as a methyl, CH3, group) from the O6 position of guanine to its active-site cysteine, thereby repairing DNA. However, AGT is S-nitrosylated in inflammatory conditions (in which iNOS is upregulated), and S-nitrosylation promotes ubiquitinylation and subsequently proteasomal degradation. GSNO is in equilibrium with SNO-AGT, and in wild-type mice, GSNOR regulates SNO-AGT denitrosylation by shifting the equilibrium towards GSNO, resulting in decreased SNO-AGT levels. However, in GSNOR−/− mice, the absence of GSNOR activity results in increased S-nitrosylation and degradation of AGT. A chromosomal deletion, inclusive of the GSNOR gene, is found in 50% of patients with HCC. Thus, GSNOR denitrosylase activity may play a tumor suppressor function in the etiology of HCC