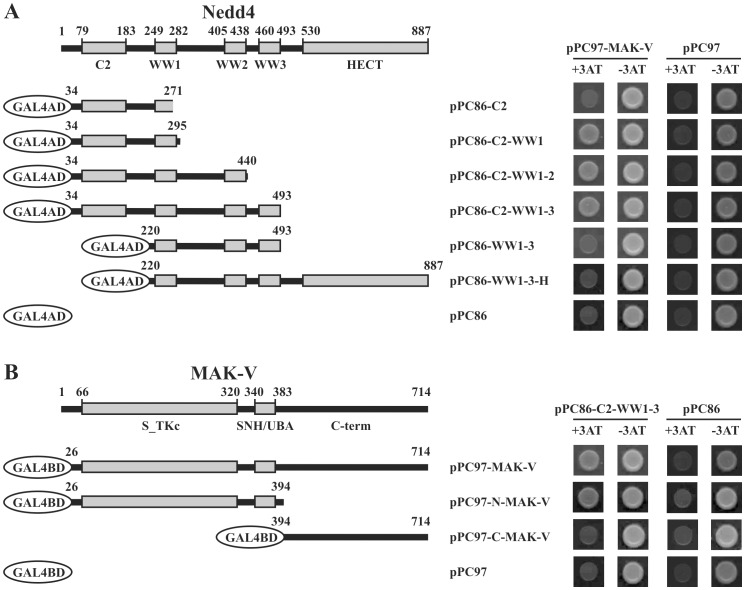
Figure 3. Mapping of interaction between Nedd4 and MAK-V proteins in yeast two-hybrid system.
(A) Domain structure of mouse Nedd4 protein showing positions (in aa) of C2, WW and HECT domains. The scheme below illustrates organization of GAL4AD proteins fused to various fragments of Nedd4, which were used in the yeast two-hybrid assay. On the right, results of analysis of interaction between GAL4BD fused to MAK-V protein (pPC97-MAK-V) and GAL4AD fused to various fragments of Nedd4 are shown. Protein interaction was monitored by ability of yeast to grow in the presence of 3AT (+3AT). To monitor specificity, interaction with GAL4BD alone (pPC97) was monitored in the same assay. (B) Domain structure of mouse MAK-V protein kinase showing positions (in aa) of catalytic (S_TKc) and SNH/UBA domains and C-terminal region (C-term). The scheme below illustrates organization of GAL4BD proteins fused to full-length of MAK-V or its N- or C-terminal fragments, which were used in the yeast two-hybrid assay. On the right, results of analysis of interaction between GAL4AD fused to fragment encompassing C2 and tree WW domains of Nedd4 (pPC86-C2-WW1-3) and GAL4AD fused to MAK-V fragments are shown. Protein interaction and specificity were monitored as described above.