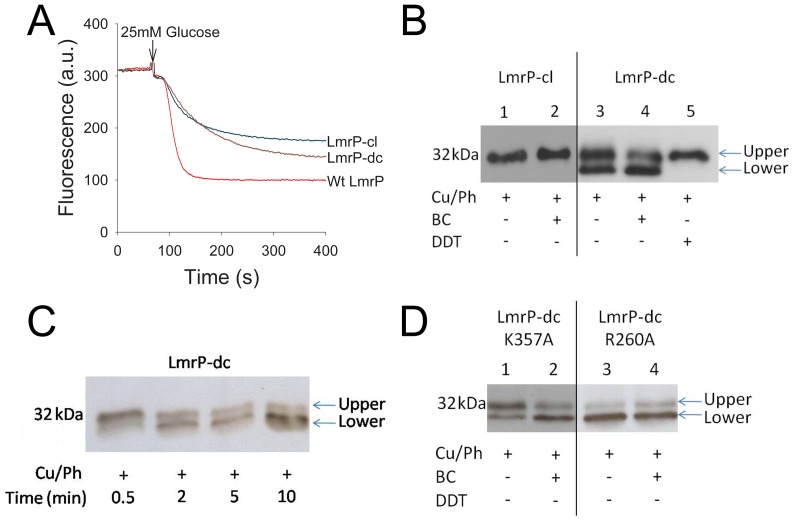
Figure 9. Intramolecular disulfide formation in LmrP-dc (I34C & V240C) and mutants derived thereof.
A, Ethidium efflux in intact cells expressing Wt LmrP, LmrP-cl or LmrP-dc show that, similar to Wt LmrP, the mutants are transport active. The measurements were performed as described in the legend to Fig. 3A. The traces represent results of three independent experiments (n = 3). B, Cysteine crosslinking between I34C and V240C in LmrP-dc. RSOVs containing LmrP-dc (2 µg protein/µl) in 100 mM KPi buffer, pH 7.0, plus 5 mM MgSO4 were exposed to 0.5 mM copper phenanthroline (Cu/Ph) in the presence or absence of 97.9 µM benzalkonium chloride (BC) for 5 min at 10°C. The reactions were stopped by the addition of excess (10 mM) of the thiol alkylator N-ethylmaleimide followed by incubation for 2 min at 10°C. Total protein (5 µg) of RSOVs from the crosslinking reactions were mixed with 5 × SDS sample-loading buffer devoid of dithiothreitol (DTT) (lane 1–4) or containing 2 mM DTT (lane 5), and separated on 11% SDS-PAGE. The SDS-PAGE gel was subsequently subjected to Western blot transfer onto a HybondP membrane. Transferred proteins were probed with anti-His5 antibody. Upper band: uncrosslinked protein; Lower band: crosslinked protein. C, Time course for disulfide formation between I34C and V240C. The crosslinking in RSOVs containing LmrP-dc was followed for 10 min at 10°C as described for panel B, lane 3. Samples of the crosslinking reactions (2 µg) were mixed with 5 × SDS sample-loading buffer devoid of DTT, and separated on SDS-PAGE. D, The effect of K357A or R260A mutation on disulfide formation between I34C and V240C. The crosslinking was performed as described in panel B for 5 min at 10°C. Equal loading of lanes was confirmed by densitometry of the same samples on Coomassie-stained SDS-PAGE (not shown).