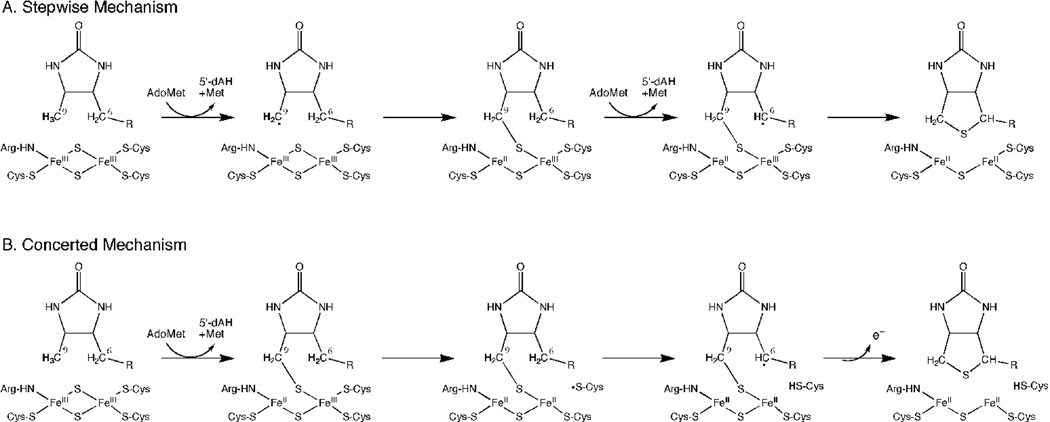
Figure 1.
Stepwise and concerted mechanisms for thiophane ring formation via a 9-MDTB intermediate. (A) In a stepwise mechanism, reductive cleavage of AdoMet is coupled to abstraction of a hydrogen atom from C9 and formation of a new C-S bond. Reductive cleavage of second equivalent of AdoMet is coupled to abstraction of a hydrogen from the C6 position, leading to completion of the thiophane ring and destruction of the [2Fe-2S]2+ cluster. 9-MDTB is formed as a stable intermediate. (B) In a concerted mechanism, initial C-S bond formation requires reductive cleavage of AdoMet and proceeds in the same manner, but a cysteine thiyl radical generated during degradation of the [2Fe-2S]2+ cluster abstracts a hydrogen atom from C6, completing the biotin thiophane ring without any additional requirement for AdoMet. One excess electron derived from sulfur oxidation could be transferred back to the catalytic [4Fe-4S]2+ cluster.