Abstract
Extramedullary plasmacytoma (EMP) is a rare plasma cell neoplasm that occurs mainly in the soft tissues of head and neck region, with the paranasal sinuses, nasal cavity and nasopharynx being the most common sites. Solitary EMP of the larynx is very rare but increasingly reported recently. Common sites of involvement in larynx in the order of frequency are the epiglottis, ventricles, vocal folds and ventricular folds. We report an extremely rare case of solitary EMP involving in the apex of arytenoids that was successfully treated by only surgical excision. Because solitary EMP of the apex of artytenoids is extremely rare, it should be included in the differential diagnosis for laryngeal mass. Also, solitary, small, pedunculated and localized EMP of the larynx could be completely removed by laryngeal microsurgery.
Keywords: Extramedullary plasmacytoma, Arytenoid, Surgical excision
INTRODUCTION
Extramedullary plasmacytoma (EMP) is a rare plasma cell neoplasm that occurs mainly in the soft tissues of head and neck region, with the paranasal sinuses, nasal cavity and nasopharynx being the most common sites (1, 2). EMPs represent 4% of all plasma cell neoplasma (3). EMP is distinct from solitary plasmacytoma of the bone and from multiple myeloma, because EMP is usually a localized disease and associated with a favorable prognosis (4).
Solitary EMP of the larynx comprising approximately six to eighteen percent of all EMPs is very rare but increasingly reported recently (5). Common sites of involvement in the larynx in the order of frequency are the epiglottis, ventricles, vocal folds and ventricular folds (6).
We report an extremely rare case of solitary EMP involving in the apex of arytenoids that was treated by only surgical excision. We review the literatures of this rare but interesting lesion and present the endoscopic, computerized tomography (CT) and pathologic findings.
CASE REPORT
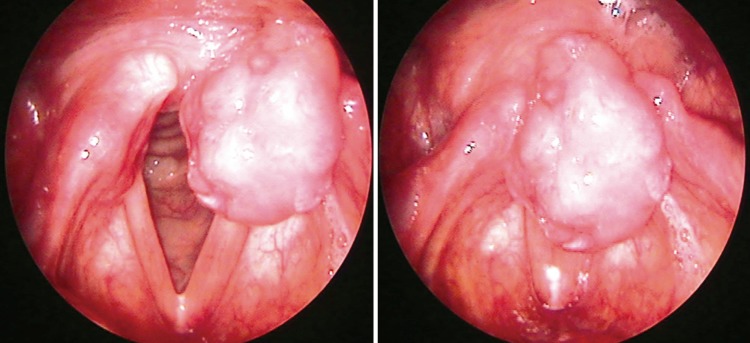
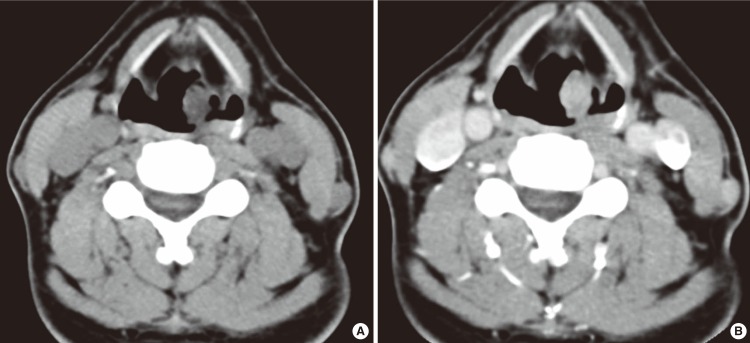
The patient was a 58-year-old healthy male who presented with a laryngeal mass incidentally discovered in the course of gastroscopy for a periodic medical check-up. He had no symptoms, including hoarseness, globus sensation, dysphagia or dyspnea. He had no previous medical history and prior history of intubation. Laryngeal examination revealed a clearly demarcated, localized mass in the apex of left arytenoid. The lesion was a reddish, polypoid growth (Fig. 1). The mobility of vocal cord was intact. No lymph node enlargement was clinically detected. CT of the neck revealed a well demarcated, minimally enhancing, 1.5×1.0×1.2 cm sized mass in the left arytenoid region (Fig. 2).
Fig. 1.
Laryngeal examination revealed a mass clearly demarcated, localized in the apex of left arytenoid. The lesion was a reddish, polypoid growth.
Fig. 2.
Computerized tomography of the neck revealed a well demarcated, minimally enhancing, 1.5×1.0×1.2 cm sized mass in the left arytenoid region.
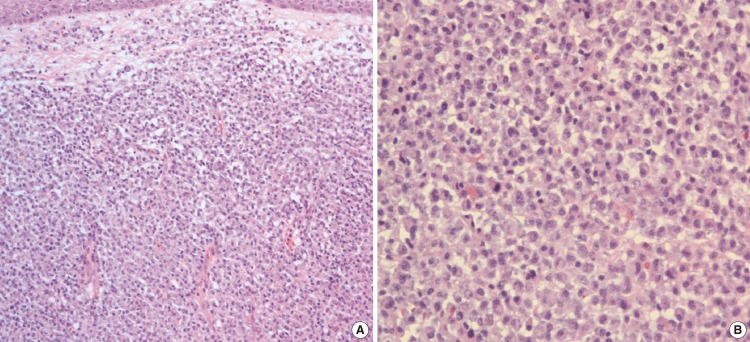
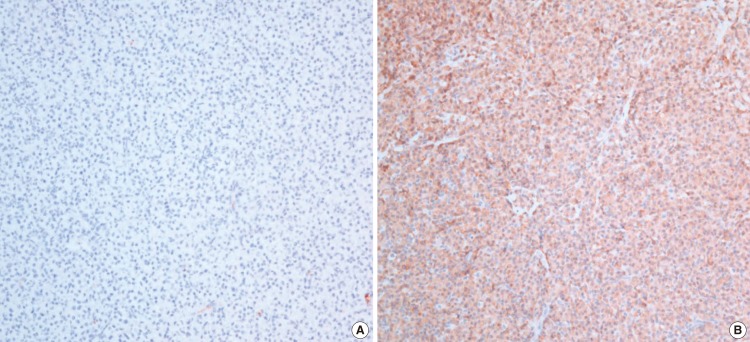
The patient underwent a direct laryngoscopy under general anesthesia. The tumor was noted to involve the apex of left arytenoid and presented as a reddish, smooth polypoidal growth with narrow base. The patient had undergone complete surgical excision with negative margins but minimal bleeding was noted. Histopathology revealed a dense monoclonal infiltrate of plasma cells with eccentrically situated nuclei with mild degree of nuclear polymorphism (Fig. 3). The histological diagnosis of plasmacytoma was made. Immunohistochemistry was done for kappa and lambda light chains and was positive for lambda chains, which confirmed the monoclonal nature of the plasma cells (Fig. 4). Multiple laboratory and radiological studies were performed in order to rule out systemic plasmacytoma. Serum protein electrophoresis, immunoelectrophoresis and scintigraphy were normal. Urine examination for light chains was negative. The full blood count and levels of serum calcium, creatinine and uric acid were within the normal range. Bone marrow aspiration from the iliac crest was normal on cytological examination and showed no evidence of plasma cell infiltration. A radiological skeletal survey was performed which showed no evidence of lytic lesions in the skull, vertebral column or bones of the chest wall or pelvis. A diagnosis of extramedullary plasmacytoma of the apex of arytenoids was made. Because the resected margin was histologically free of tumor cells, no further therapy including adjuvant radiotherapy was administered. He has remained clear, with no local recurrence during 2 years of follow-up.
Fig. 3.
Histopathology revealed a dense monoclonal infiltrate of plasma cells with eccentrically situated nuclei with mild degree of nuclear polymorphism (A: ×200, B: ×400).
Fig. 4.
Immunohistochemistry was done for kappa (A) and lambda light chains (B) and was positive for lambda chains, which confirmed the monoclonal nature of the plasma cells (×200).
DISCUSSION
EMPs constitute less than 1% of the malignancies in the head and neck region and less than 0.2% of the malignancies in the larynx (7, 8). In the present case, the apex of arytenoids is extremely rare involved site and there are few reported cases in English literatures (9).
EMP of the larynx occurs in men approximately three times more often than in women, and is generally seen at the age of 40-70 years (3, 6, 10).
Although the clinical presentation varies according to the involved organ (3), the clinical appearance of EMP of the larynx is not specific and related to compression and obstruction of local structures (11). The most common symptom is slowly progressive hoarseness. Late symptoms are dysphagia, stridor, and pain associated with locally aggressive tumors (5). Acute airway obstruction requiring intubation is an extremely rare event and may represent hemorrhage within the tumor or secondary bacterial infection (12).
In the present case, he had no clinical presentation and the lesion was incidentally discovered in the course of gastroscopy. We think that EMPs of the apex of arytenoids could be free of symptom and incidentally discovered.
The diagnosis of EMP is confirmed by histopathologically proven proliferation of neoplastic plasma cells and systemic examinations that rule out bone involvement through bone surveys, chest X-rays, peripheral blood tests and bone marrow biopsies (13).
According to the laryngoscopic view, EMPs of the larynx arise in the submucosa and vary from smooth polypoidal growths with narrow bases to sessile lesions with wide attachments. The tumors may be red or pale pink. These tumors are reportedly vascular and bleed readily with operative trauma (10). In the present case, the tumor arose in the apex of left arytenoid and presented as a red, polypoid growth with narrow base. Also, the tumor was not vascular and minimal bleeding was noted during complete excision.
No pathognomonic or characteristic radiological findings of EMP involved in the larynx have been reported since. Although our patient was not a typical case, neck CT with contrast enhancement was performed and showed well demarcated and minimally enhancing mass in the left arytenoid.
EMPs of the larynx should be differentiated from the laryngeal malignancies and benign tumors, including laryngeal granuloma and papilloma in view of laryngoscopic findings. Laryngeal granulomas, in particular, should be included in the differential diagnosis for solitary EMP involved in the arytenoid area like our case because there are several points of similarity between the two (14).
First, they are somewhat similar in the involved site. Laryngeal granulomas are benign lesions of the posterior glottis that are commonly centered in the tips of the cartilaginous vocal processes of the arytenoids. Second, laryngoscopic findings of laryngeal granuloma are also pale, sometimes red mass-like lesion and may appear as solitary or bilobed. Third, both diseases commonly present with hoarseness.
However, there is an important difference between them in points of treatment. The primary management of laryngeal granuloma is management of the underlying laryngopharyngeal reflux. Most granulomas resolve with medical management. Although surgical biopsy can be a differential diagnostic method, surgical excision may be considered for laryngeal granuloma under three conditions: 1) for biopsy, when the possibility of carcinoma exists; 2) for airway obstruction, if the granulomas are quite large; 3) to restore the voice in selected cases (14).
There are no general guidelines for the treatment of patients with EMP. The rarity of this tumor and its long natural history make determination of the optimal treatment strategy and prognostic factors difficult, which has resulted in a lack of consensus on how best to manage this condition (11). However, Radiotherapy is accepted as the standard treatment for EMP because of its high radiosensitivity. The optimal dosage regimen for radiotherapy is a matter of debate (7). Although EMP of the larynx should be treated basically by radiotherapy, surgical resection is beneficial in the treatment of small EMP of the larynx. Thus, small, pedunculated and localized lesions could be completely removed by laryngeal microsurgery (9, 10). The larger lesions, however, necessitating major surgery and sacrificing laryngeal function, are best treated by radiotherapy (9).
In the present case, because the tumor was small and localized in the apex of left arytenoid, our patient had undergone only complete surgical excision with negative margins instead of recommendation for adjuvant radiotherapy.
In general, EMP of the head and neck has a better prognosis than solitary bone plasmacytoma or multiple myeloma. This may be due to early presentation, when the lesions are small and well localized and also probably due to easy accessibility of the tumor to examination (7, 11). Because the small number of patients with EMP of the larynx was reported, the prognosis has not been reported, but the results of treatment were always good.
The evolution of EMP to multiple myeloma is the determinant factor for survival. Only 10% to 30% of cases progress to multiple myeloma within 2 years, but the prognosis for these patients is poor (15). Several factors predictive of progression to multiple myeloma have been reported, such as the size of the tumor at diagnosis, total serum protein level, and a monoclonal spike observed on serum protein electrophoresis (16). Because recurrence is usually within 24 months but may be as late as 10 years after treatment, long-term follow-up, including not only local control but also systemic observation via blood counts and immunoglobulin measurement, is necessary (7, 17).
Footnotes
No potential conflict of interest relevant to this article was reported.
References
- 1.Horny HP, Kaiserling E. Involvement of the larynx by hemopoietic neoplasms: an investigation of autopsy cases and review of the literature. Pathol Res Pract. 1995 Mar;191(2):130–138. doi: 10.1016/S0344-0338(11)80562-5. [DOI] [PubMed] [Google Scholar]
- 2.Kapadia SB, Desai U, Cheng VS. Extramedullary plasmacytoma of the head and neck: a clinicopathologic study of 20 cases. Medicine (Baltimore) 1982 Sep;61(5):317–329. doi: 10.1097/00005792-198209000-00004. [DOI] [PubMed] [Google Scholar]
- 3.Medini E, Rao Y, Levitt SH. Solitary extramedullary plasmacytoma of the upper respiratory and digestive tracts. Cancer. 1980 Jun;45(11):2893–2896. doi: 10.1002/1097-0142(19800601)45:11<2893::aid-cncr2820451130>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- 4.Kayrouz T, Jose B, Chu AM, Scott RM. Solitary plasmacytoma. J Surg Oncol. 1983 Sep;24(1):46–48. doi: 10.1002/jso.2930240111. [DOI] [PubMed] [Google Scholar]
- 5.Kost KM. Plasmacytomas of the larynx. J Otolaryngol. 1990 Apr;19(2):141–146. [PubMed] [Google Scholar]
- 6.Pahor AL. Plasmacytoma of the larynx. J Laryngol Otol. 1978 Mar;92(3):223–232. doi: 10.1017/s0022215100085261. [DOI] [PubMed] [Google Scholar]
- 7.Maclennan KA, Schofield JB. Heamopoeitic neoplasms. In: Ferlito A, editor. Neoplasms of the larynx. Edinburg: Churchill Livingstone; 1993. pp. 331–333. [Google Scholar]
- 8.Cady B, Rippey JH, Frazell EL. Non-epidermoid cancer of the larynx. Ann Surg. 1968 Jan;167(1):116–120. doi: 10.1097/00000658-196801000-00019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nakashima T, Matsuda K, Haruta A. Extramedullary plasmacytoma of the larynx. Auris Nasus Larynx. 2006 Jun;33(2):219–222. doi: 10.1016/j.anl.2005.11.019. [DOI] [PubMed] [Google Scholar]
- 10.Gorenstein A, Neel HB, Devine KD, Weiland LH. Solitary extramedullary plasmacytoma of the larynx. Arch Otolaryngol. 1977 Mar;103(3):159–161. doi: 10.1001/archotol.1977.00780200085009. [DOI] [PubMed] [Google Scholar]
- 11.Bachar G, Goldstein D, Brown D, Tsang R, Lockwood G, Perez-Ordonez B, et al. Solitary extramedullary plasmacytoma of the head and neck: long-term outcome analysis of 68 cases. Head Neck. 2008 Aug;30(8):1012–1019. doi: 10.1002/hed.20821. [DOI] [PubMed] [Google Scholar]
- 12.Gormley PK, Primrose WJ, Bharucha H. Subglottic plasmacytoma of the larynx: an acute presentation. J Laryngol Otol. 1985 Sep;99(9):925–929. doi: 10.1017/s0022215100097942. [DOI] [PubMed] [Google Scholar]
- 13.Batsakis JG. Pathology consultation: plasma cell tumors of the head and neck. Ann Otol Rhinol Laryngol. 1983 May-Jun;92(3 Pt 1):311–313. doi: 10.1177/000348948309200320. [DOI] [PubMed] [Google Scholar]
- 14.Koufman JA, Halum SL, Postma GN. Controversies in laryngology. In: Bailey BJ, Johnson JT, Newlands SD, Calhoun KH, Deskin RW, editors. Head and neck surgery-otolaryngology. 4th ed. Philadelphia: Lippincott Williams & Wilkins; 2006. pp. 910–911. [Google Scholar]
- 15.Husain M, Nguyen GK. Primary pulmonary plasmacytoma diagnosed by transthoracic needle aspiration cytology and immunocytochemistry. Acta Cytol. 1996 May-Jun;40(3):622–624. [PubMed] [Google Scholar]
- 16.Holland J, Trenkner DA, Wasserman TH, Fineberg B. Plasmacytoma: treatment results and conversion to myeloma. Cancer. 1992 Mar;69(6):1513–1517. doi: 10.1002/1097-0142(19920315)69:6<1513::aid-cncr2820690633>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- 17.Yavas O, Altundag K, Sungur A. Extramedullary plasmacytoma of nasopharynx and larynx: synchronous presentation. Am J Hematol. 2004 Apr;75(4):264–265. doi: 10.1002/ajh.20038. [DOI] [PubMed] [Google Scholar]