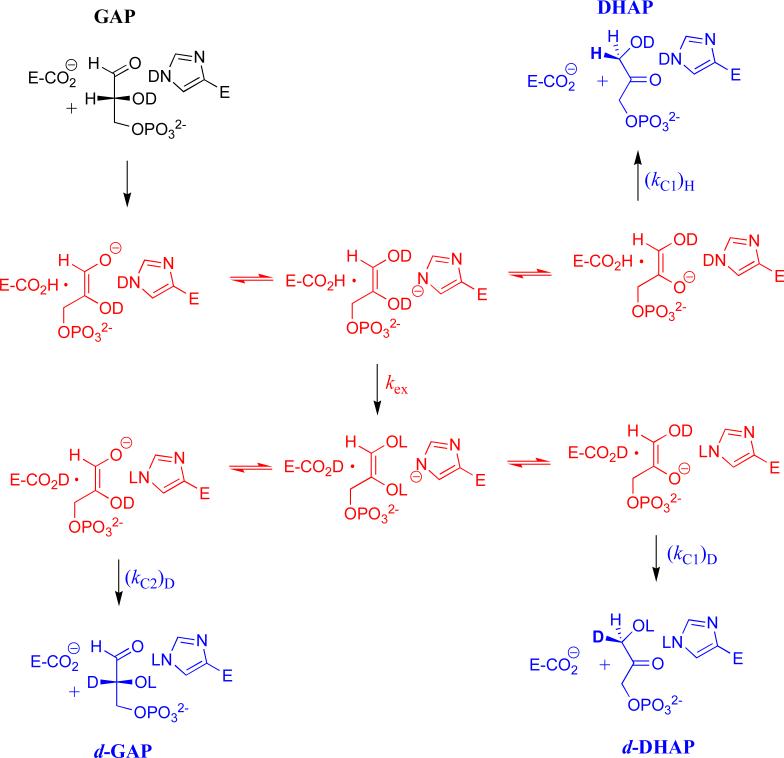
Figure 4.
A minimal mechanism to rationalize the yields of the products of wildtype and mutant TIM-catalyzed reactions of GAP in D2O. The -H derived from substrate is exchanged with a pool of two -D at the enzyme, the intermediate –OD and the -D at N-3 of the imidazole side chain of His-95. Fast transfer between four basic sites; the carboxylate of Glu-165, the two oxygen anions of the isomeric intermediates, and the N-3 imidazolate of His-95 scrambles a total of three hydrons. The steps that result in scrambling are not shown, but are subsumed in the macroscopic rate constant kex. A mechanism, referred to as the criss-cross mechanism, has been proposed to explain the effects of the H95Q mutation on the product yields for reactions of GAP in 3H-labeled water.12 In this mechanism (not shown) the -H derived from substrate exchanges with a single hydron at the intermediate –OD, and there is no scrambling of hydrons at the C-1 and C-2 hydroxyl groups of the intermediate. In cases where a >2/3 yield of deuterium labeled products is obtained, the substrate-derived hydron either undergoes fast irreversible exchange with deuterium at TIM, or reversible exchange that scrambles a pool of >2 -D at the enzyme active site.50