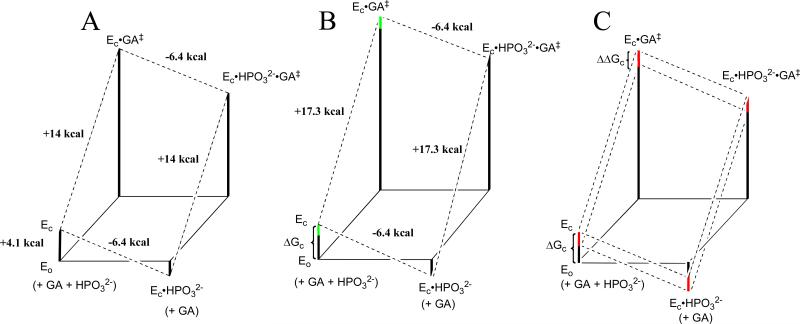
Figure 5.
Free energy profiles for turnover of GA by free TIM (EO) and by TIM that is saturated with HPO32- (EC•HPO32-) constructed using the kinetic parameters reported in Table 5. The profiles show the activation free energy changes calculated using the Eyring equation at 298 K for reactions catalyzed by wildtype and mutant forms of TbbTIM. (A) Reactions catalyzed by wildtype TbbTIM. The difference between the total intrinsic phosphite binding energy of -6.4 kcal/mol and ΔG° = -2.3 kcal/mol for binding of HPO32- to the inactive open enzyme EO to give the active closed liganded enzyme EC•HPO32- is attributed to ΔGC = 4.1 kcal/mol for the conformational change that converts EO to EC. (B) Reactions catalyzed by I172A mutant TbbTIM. The overall barrier for conversion of EC•HPO32- to the transition state for reaction of GA is 3.3 kcal/mol higher than for the reaction catalyzed by wildtype TbbTIM. The green bars show the uncertainty in the barrier to the unactivated reaction of GA, that was drawn using the upper limit of (kcat/Km)E < 0.003 M-1 s-1 (Table 5) and a lower limit calculated with the assumption that the total 6.4 kcal/mol intrinsic phosphate dianion binding energy is equal to that for wildtype TbbTIM. (c) Reactions catalyzed by L232A mutant TbbTIM. The red bars show the proposed effect of the L232A mutation on the barrier for the conformational change from EO to EC (ΔΔGC). A comparison of the reaction profiles for wildtype TIM (upper dashed lines) and the L232A mutant (lower dashed lines) shows the effect of this change in ΔGC on the kinetic parameters for the reaction of the substrate pieces.