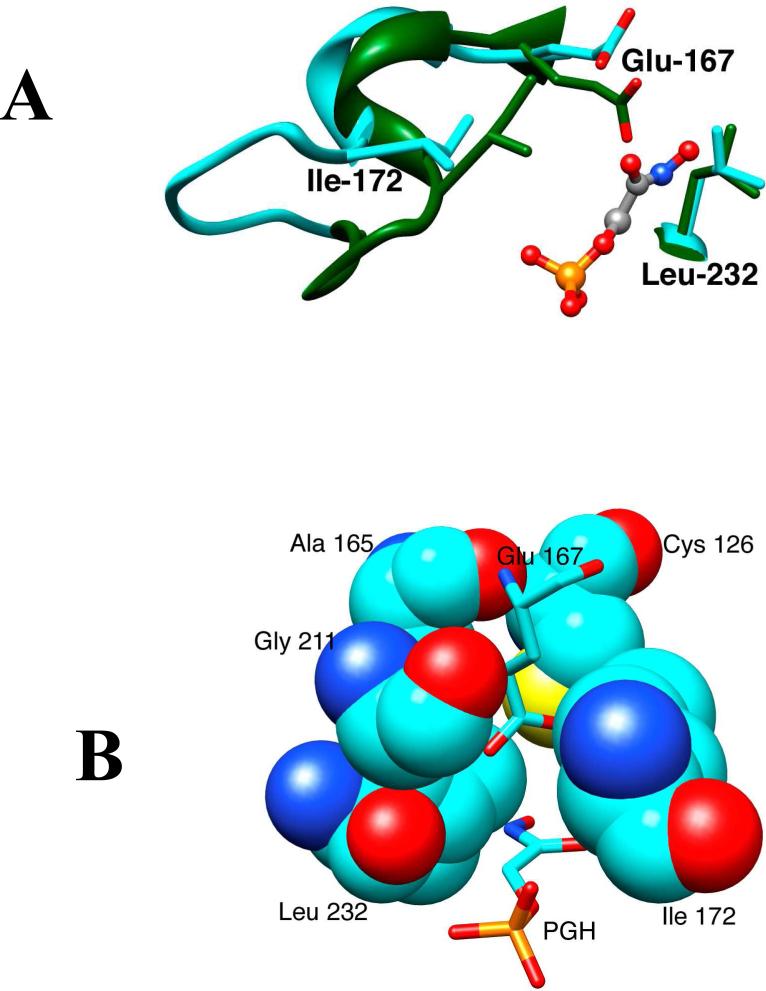
Figure 2.
(A) Models from X-ray crystal structures of the unliganded open (cyan, PDB entry 5TIM) and PGH-liganded closed (green, PDB entry 1TRD) forms of TIM from Trypanosoma brucei brucei in the region of the enzyme active site. Closure of loop 6 (residues 168 – 178, Scheme 2) over the bound phosphodianion ligand results in movement of the hydrophobic side chain of Ile-172 toward the carboxylate side chain of the catalytic base Glu-167. This is accompanied by movement of Glu-167 toward the hydrophobic side chain of Leu-232, that maintains a nearly fixed position. (B) A structure that shows the positions of Ile-172 and Leu-232 at the active site “hydrophobic cage” for wildtype Trypanosoma brucei TIM in complex with PGH (PDB entry 1TRD).