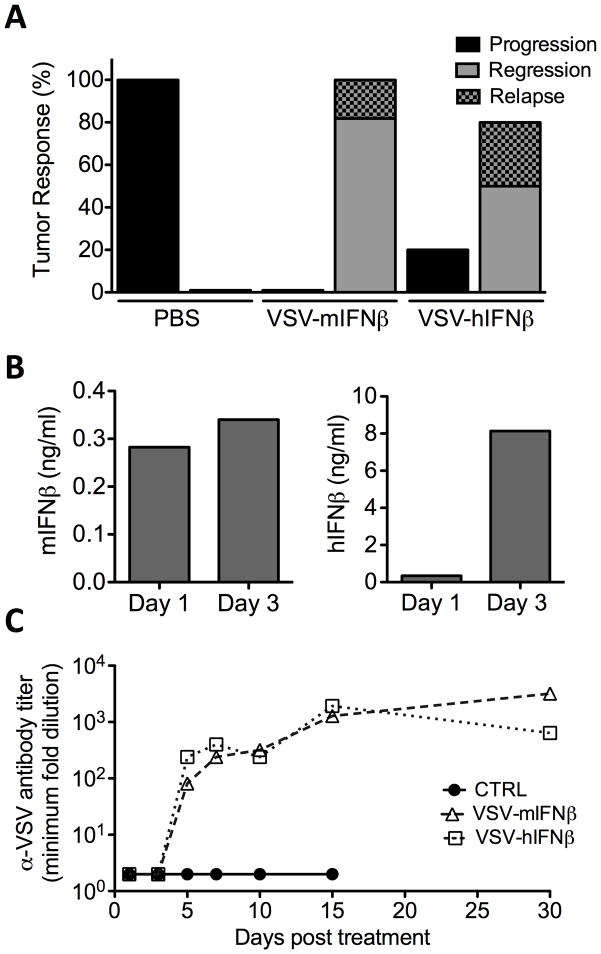
Figure 2. VSV-IFN oncolytic activity against 5TGM1 myeloma tumors in syngeneic immune competent mice.
Female, 6–8 week old C57Bl/KaLwRij mice bearing subcutaneous syngeneic 5TGM1 myeloma tumors (~200mm3) were treated with single intravenous (IV) dose of 100ul PBS, or 1×108TCID50 VSV-mIFNβ or VSV-hIFNβ. (A) Tumor responses following IV treatment was monitored by serial caliper measurements and categorized into tumor progression (black), regression (grey), or regression with relapse (shaded grey). Tumor responses are shown as percentage of mice in each treatment group. (B) In vivo IFNβ expression following treatment with VSV-mIFNβ (red) or VSV-hIFNβ (blue) was measured in serum harvested at day 1 and 3 post treatment assayed by ELISA for murine IFNβ or human IFNβ respectively. (C) α-VSV neutralizing antibodies in serum of mice treated with PBS (black), VSV-mIFNβ (red) or VSV-hIFNβ (blue). Serial 2-fold dilutions of heat-inactivated serum pre-incubated with VSV-GFP and subsequently used to infect Vero cells. Measured antibody titer is the minimum serum dilution that fails to protect against VSV induced CPE. Serum was harvested from n=2 mice per groups at each time point, except where only one mouse was surviving in the treatment group.