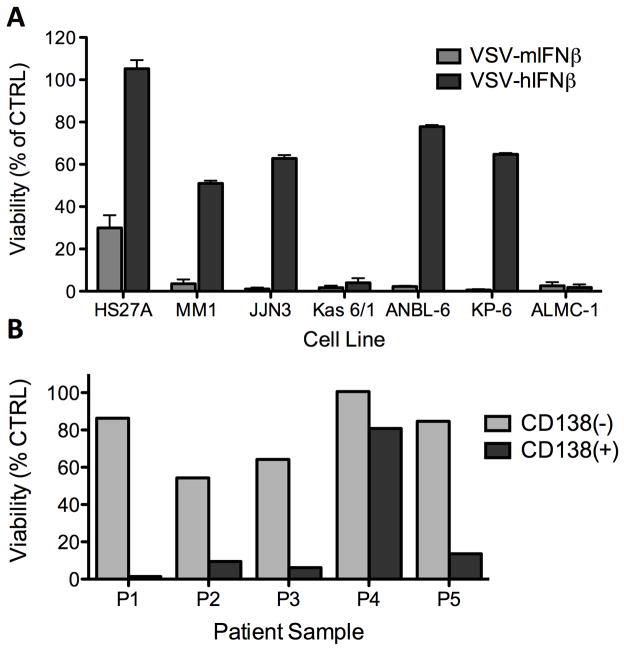
Figure 6. In vitro activity of recombinant VSV-IFN in human myeloma cell lines.
(A) Variable cytopathic activity of VSV-mIFNβ vs. VSV-hIFNβ in a panel of human myeloma cell lines. Oncolytic activity was determined by MTT assay at 72h post infection of myeloma cell lines at MOI 1.0. Results are plotted as percentage of mock-infected cells (B) VSV-hIFNβ cytopathic activity in primary myeloma samples from 5 separate patients. Patient bone marrow aspirates sorted into CD138(+) myeloma (grey) and CD138(−) non myeloma cells (shaded) infected with VSV-hIFNβ (MOI 1.0). Cell viability was assessed at 48h post infection by flow cytometry analysis.