Abstract
Interaction of estrogen with iron at the systemic level is long suspected, but direct evidence linking the two is limited. In the present study, we examined the effects of 17β-estradiol (E2) on hepcidin, a key negative regulator of iron absorption from the liver. We found that transcription of hepcidin was suppressed by E2 treatment in human liver HuH7 and HepG2 cells, and this down-regulation was blocked by E2 antagonist ICI 182780. Chromatin immunoprecipitation, deletion, and EMSA detected a functional estrogen responsive element half-site that is located between −2474 and −2462 upstream from the start of transcription of the hepcidin gene. After cloning the human hepcidin promoter into the pGL3Luc-Reporter vector, luciferase activity was also down-regulated by E2 treatment in HepG2 cells. E2 reduced hepcidin mRNA in wild-type mice as well as in hemochromatosis Fe gene knockout mice. In summary, our data suggest that hepcidin inhibition by E2 is to increase iron uptake, a mechanism to compensate iron loss during menstruation. This mechanism may also contribute to increased iron stores in oral contraceptive users.
Osteoporosis and breast cancer are two most common diseases that affect women's health. Estrogen deficiency as a result of menopause is considered the main cause of postmenopausal osteoporosis (1). Highly accumulated exposure to estrogens due to early menarchy, late menopause, and nulliparity is a well-established risk factor for breast cancer (2). However, studies on clinical outcomes, disease characteristics, and molecular mechanisms indicate that development of osteoporosis and breast cancer cannot be solely explained by changes in estrogens alone (3, 4). Variations in iron levels from deficiency in young women to oversufficiency in older postmenopausal women have recently been postulated to play important roles in the two diseases (3, 4).
Iron is essential for fundamental metabolic processes in cells and organisms. Similar to estrogens, disruptions in iron homeostasis from both iron deficiency and overload account for some of the most frequent human diseases (5). Iron deficiency anemia is a widespread malady in young women (6). Indeed, we have shown that iron deficiency increases hypoxia-inducible factor-1α, vascular endothelial growth factor, and angiogenesis (7). These results suggest that systemic iron deficiency might contribute to breast tumor malignancy and high recurrence rate in this young age group of breast cancer patients (8, 9). On the other hand, we have found that increased iron inhibits preosteoblast cell differentiation and suppresses alkaline phosphatase activities in maturing osteoblasts (10). These data imply that in addition to increased bone resorption caused by estrogen deficiency, increased iron could be a factor that slows down bone formation in postmenopausal women.
Menstruation and insufficient dietary iron uptake are considered main causes of iron deficiency in young women. On the other hand, cessation of menstruation is viewed as a main cause of iron accumulation in older postmenopausal women. We have previously observed that although levels of 17β-estradiol (E2) are high in young women, levels of iron in the form of ferritin are low (Supplemental Fig. 1, published on The Endocrine Society's Journals Online web site at http://endo.endojournals.org) (4). In older postmenopausal women, the reverse is true, with low E2 and high iron levels. These concurrent but inverse changes in E2 and iron levels during menopausal transition prompted us to investigate whether changes in E2 levels could lead to changes in iron levels. In the present study, we investigated whether E2, the most active form of estrogens, affects expression of hepcidin, a key iron regulatory hormone, and, thus, may alter body iron homeostasis.
Hepcidin is a 25-amino acid peptide produced by hepatocytes (11). It binds to ferroportin, an iron exporter, and causes internalization (12). Hepcidin inhibits intestinal iron absorption by blocking ferroportin to transport iron from the gastrointestinal system to the circulatory system. It prevents iron release from ferroportin-containing cells, such as macrophages and recycling senescent erythrocytes, resulting in iron accumulation in local tissues, such as fat (13). Hepcidin transcription can be up-regulated by iron, IL-6, IL-1α, IL-1β, and bone morphogenetic proteins and down-regulated by anemia and hypoxia (14, 15). In this study, we have identified a functional estrogen responsive element (ERE) half-site in the promoter region of hepcidin gene. The role of E2 appears to inhibit hepcidin and to increase iron uptake, a mechanism to compensate iron loss during menstruation.
Materials and Methods
Cell culture
Cell culture reagents were purchased from Invitrogen (Carlsbad, CA) unless otherwise stated. The human liver cell lines, HuH7 and HepG2, both having male DNA, were obtained from American Type Culture Collection (Manassas, VA). They were maintained in α-MEM containing 10% charcoal/dextran-stripped fetal bovine serum, penicillin (100 U/ml), streptomycin (100 μg/ml), and L-glutamine (2 mm). They were grown at 37 C in a humidified atmosphere containing 5% CO2.
Plasmids cloning and deletion
The human hepcidin promoter (−3038/+21) was cloned from human genome (Promega, Madison, WI) into a pGL3-basic reporter plasmid. The pGL3 plasmid containing ERE-deleted hepcidin mutant was constructed by a QuikChange Site-Directed Mutagenesis kit (Stratagene, Santa Clara, CA). Primer sequences used for this study are listed in Table 1, and all of the constructs were verified by sequencing.
Table 1.
The primer sequences of the oligonucleotides used for ChIP, RT-PCR, qRT-PCR, and cloning
| Name | Gene ID | Application | Sequences |
|---|---|---|---|
| Human hepcidin, first from the left of Fig. 1A | 57817 | ChIP | Forward, 5′-CAGTGGTTGGTTGGATGGAT |
| Reverse, 5′-TTGTGGGAAGAGACGAGTGC | |||
| Human hepcidin second site | 57817 | ChIP | Forward, 5′-AACCGGCTCCCCAAAAAC |
| Reverse, 5′-CCCCGTCGGACACTCATT | |||
| Human hepcidin third site | 57817 | ChIP | Forward, 5′-CGCCGTGTGGAGTCTTATTC |
| Reverse, 5′-GCTTATGGGGGCTTCCTCT | |||
| Human hepcidin fourth site | 57817 | ChIP | Forward, 5′-GTCCCCTCCCTTCCTTATTT |
| Reverse, 5′-CTTTCATCCCCTATTATTGCAAC | |||
| Human hepcidin | 57817 | RT-PCR | Forward, 5′-CCTGACCAGTGGCTCTGTTT |
| Reverse, 5′-CACATCCCACACTTTGATCG | |||
| Human GAPDH | 2597 | RT-PCR | Forward, 5′-TGGTATCGTGGAAGGACTC |
| Reverse, 5′-AGTAGAGGCAGGGATGATG | |||
| Mouse GAPDH | 14433 | qPCR | Forward, 5′-GGCATTGCTCTCAATGACAA |
| Reverse, 5′-CCCTGTTGCTGTAGCCGTAT | |||
| Mouse hepcidin-1 | 84506 | qPCR | Forward, 5′-AGAAAGCAGGGCAGACATTG |
| Reverse, 5′-GATGCAGATGGGGAAGTTGG | |||
| Mouse hepcidin-2 | 66438 | qPCR | Forward, 5′-AGAAAGCAGGGCAGACATTG |
| Reverse, 5′-TCTGCAGATGGGGAAGTTGA | |||
| Human hepcidin promoter (3171 bp) | 57817 | Luciferase activity | Forward, 5′-TCTCGAGCTCAAGCTTGTCTGTCTGTCGCCCTTCTC |
| Reverse, 5′-GGCGACCGGTGGATCCGTCTGGGACCGAGTGACAGT | |||
| Human hepcidin promoter | 57817 | ERE deletion | Forward, 5′-AGGGTGTGGGGAGCTGGAGGACATGTCCCATGTTG |
| Reverse, 5′-CAACATGGGACATGTCCTCCAGCTCCCCACACCCT | |||
| Human hepcidin probe with WT ERE | 57817 | EMSA | Forward, 5′-AGGGTGTGGGGAGCTGGGGTCAAGGACATGTCCCATGTTG |
| Reverse, 5′-CAACATGGGACATGTCCTTGACCCCAGCTCCCCACACCCT | |||
| Human hepcidin probe with mutated ERE | 57817 | EMSA | Forward, 5′-AGGGTGTGGGGAGCTGGAAAAAAGGACATGTCCCATGTTG |
| Reverse, 5′-CAACATGGGACATGTCCTTTTTTCCAGCTCCCCACACCCT | |||
| TfR1 | 22042 | qPCR | Forward, 5′-CATGAGGGAAATCAATGATCG |
| Reverse, 5′-ACATAGGGCGACAGGAAGTG | |||
| DMT-1 | 18174 | qPCR | Forward, 5′-TGGGTCTGTCTTTCCTGGAC |
| Reverse, 5′-CATCCACGGTGTTCAGAAGAT |
RNA isolation and PCR analyses
Human liver HuH7 and HepG2 cells were cultured on six-well plates until 80% confluency. After starving in serum-free α-MEM for 24 h, cells were pretreated with ICI 182780 at 1 μm for 0.5 h and then treated with E2 (Sigma, St. Louis, MO) at 100 nm for 4 and 24 h, respectively. Total RNA was extracted using TRIzol (Invitrogen). The first-strand cDNA was synthesized with 1 μg of the total RNA using the ThermoScript Reverse Transcription kit (Invitrogen). Two PCR techniques were used in this study: RT-PCR and quantitative real-time PCR (qPCR). Using one twentieth of the cDNA as a template, the RT-PCR was carried out for each transcript under these conditions: 15–25 cycles of 94 C for 30 sec, 58 C for 30 sec, and 72 C for 30 sec to determine the exponential phase of amplification. As an internal control, amplification of glyceraldehyde-3-phosphate dehydrogenase (GAPDH) mRNA was carried out with similar cycle optimization.
The cDNA, used as template for qPCR, was mixed with SYBR Green Supermix (Bio-Rad, Hercules, CA) and the primers of the target genes (Table 1). The qPCR was run in a 384-well plate (ABI 7900 series; Applied Biosystems, Foster City, CA). The mRNA expression levels of the target genes were first normalized to the housekeeping gene, GAPDH. Data were then expressed as fold change over the untreated control.
Luciferase assays
To examine the promoter activities in response to water soluble E2 HepG2 cells were grown at a density of 1 × 105 cells/ml in 24-well plates and cotransfected with 0.8 μg of the luciferase constructs, 50 ng of Renilla luciferase plasmid by Lipofectamine 2000 (Invitrogen). After 24 h, cells were starved with serum-free α-MEM for 24 h and treated with E2 for various times. Relative luciferase activities were first normalized to Renilla and then expressed as fold change over the untreated control. Each experiment was performed in triplicate and repeated at least three times.
Chromatin immunoprecipitation (ChIP)
HuH7 cells starved in serum-free MEM overnight were treated with 100 nm E2 for 24 h, and ChIP assay was carried out following the manufacturer's instructions (Upstate, Lake Placid, NY). In brief, formaldehyde cross-linking was quenched by adding glycine. The cell lysates were sonicated to shear DNA and further diluted in ChIP dilution buffer. To reduce nonspecific binding, the cell pellet suspension was precleared with salmon sperm DNA/protein A agarose-50% slurry. ChIP assays were performed overnight at 4 C with antibodies against estrogen receptor (ER)α (EMD Millipore, Billerica, MA) or normal mouse IgG (Santa Cruz Biotechnology, Inc., Santa Cruz, CA). Protein A agarose-50% slurry was added to collect the antibody/histone complex. Input and immunoprecipitated chromatin were incubated at 65 C overnight to reverse cross-links. After proteinase K digestion, DNA was extracted using a spin column kit (QIAGEN, Valencia, CA). An aliquot of each sample was assayed by PCR. All primers used for this study are listed in Table 1. The PCR product was electrophoresed on a 2% agarose gel and visualized by ethidium bromide staining.
Electrophoretic mobility shift assay
HepG2 cells were treated with E2 at 100 nm for 24 h. Nuclear extracts were prepared using NE-PER nuclear extraction reagent (Pierce, Rockford, IL). Oligonucleotides containing the wild-type (WT) and the mutant ERα binding site within the hepcidin promoter were biotin labeled using a biotin 3′-end DNA labeling kit (Pierce). Equal amounts of labeled and complementary oligonucleotides were gradually allowed to cool to room temperature to allow the annealing of double-stranded oligonucleotides. EMSA was performed according to the LightShift Chemiluminescent EMSA kit (Pierce). Briefly, 10 μg of the nuclear protein extracts were incubated with 20 fmol biotin-labeled oligonucleotides for 20 min at room temperature. Competitive reaction was performed under identical condition by adding 200-fold of the amount of unlabeled oligonucleotides. For further identification of the complexes by a supershift assay, the anti-ERα antibody was preincubated with the nuclear extract on ice for 30 min before EMSA. Normal IgG was used as the control. Samples were separated by nondenaturing 5% polyacrylamide gel electrophoresis and transferred to Hybond-N+ nylon membrane (GE Healthcare, Piscataway, NJ). The membrane was blocked and applied with streptavidin-horseradish peroxidase conjugate for 15 min. After thorough washing, the membrane was exposed to x-ray film after incubation with the chemiluminescence reagents.
In vivo experiment
All animal studies were approved by our Institutional Animal Care and Use Committee. Eight-week-old female 129/SvEv mice (The Jackson Laboratory, Bar Harbor, ME) and hemochromatosis Fe (Hfe) gene knockout mice with the same genetic background (a gift from N. C. Andrews, Duke University, Durham, NC) were randomized and then ip injected with 100 nmol E2/kg bodyweight. The mice were allowed unrestricted activity and food and water ad libitum. After sacrificing the mice 24 h later, liver and bone marrow samples were isolated, and blood samples were collected by heart puncture. The sera were analyzed for serum iron in WT mice using ferrozine assay (16). RNA from the liver and from bone marrow of the tibia and femora were extracted. qPCR was used for measurements of hepcidin mRNA (hepcidin-1 and hepcidin-2) in liver and transferrin receptor-1 (TfR1) and divalent metal transporter-1 (DMT-1) mRNA in bone marrow (Table 1). Data were expressed as fold change over control after normalizing to the housekeeping gene, GAPDH.
Statistical analyses
Statistical evaluations of the data were conducted by Student's t test for paired comparison or by one-way ANOVA for multiple comparisons, followed by a post hoc Newmann-Keuls test. The results were presented as means ± sd, and P values less than 0.05 were considered to be significantly different.
Results
Prediction of ERE in the promoter region of hepcidin promoter
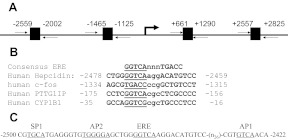
The canonical ERE has a 13-base sequence of GGTCAnnnTGACC where nnn are known as the tri-nucleotide spacer (17). We examined the sequence of the 5′-flanking region of the hepcidin gene and found four ERE in the promoter using the web-based Genomatix/MatInspector and TESS software (Fig. 1A). Sequence analyses show that one region of hepcidin gene contains a consensus ERE half-site (GGTCA) at −2474 with an imperfect half-site (ACATG) at −2462 (Fig. 1A). This sequence is highly similar to the ERE found in genes for human c-Fos (18), pituitary tumor transforming gene (PTTG) binding factor (PTTG1IP) (19), and CYP1B1 genes (20), as shown in Fig. 1B. To explain the functionality of this imperfect consensus ERE, it has been shown that approximately 50% of all ERα-bound loci do not have a discernible ERE, and most ERα-bound ERE are not perfect consensus ERE (21). ERα dimers are known to bind to ERE half-site (22, 23), although binding can be enhanced by other transcription factors, such as specificity protein-1 (SP-1) or activator protein 1 (AP-1) (24). Further analyses display that the same region indeed contains SP-1 and AP-1/2-sequence elements (Fig. 1C).
Fig. 1.
Prediction of ERE in the promoter region of the hepcidin gene. A, The human hepcidin promoter region contains four ERE. B, Comparison of the ERE sequence of the hepcidin gene with the consensus sequence of human c-Fos, PTTG1IP, and CYP1B1 genes. The invariant sequence is underlined. C, Illustration of ERE half-site, SP-1, and AP-1/2-binding site sequences in human hepcidin promoter region between −2500 and −2422. Nucleotide numbers are denoted with the transcription start site assigned as +1.
Inhibition of hepcidin mRNA expression by estrogen treatment
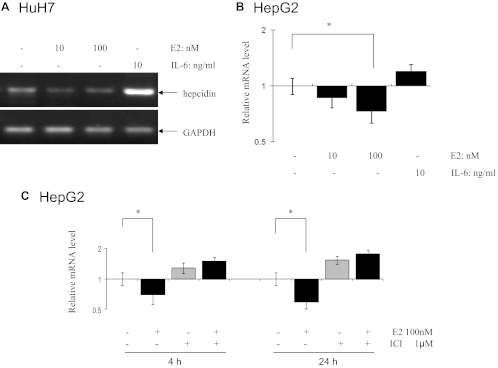
To assess estrogen-mediated hepcidin regulation, human liver HuH7 and HepG2 cells were grown in a medium containing charcoal/dextran-stripped serum for 3 wk before E2 treatments. Semiquantitative RT-PCR and qPCR were performed in HuH7 and HepG2 cells, respectively. GAPDH was used as a internal loading control and IL-6 as positive control for hepcidin induction (25). As shown in Fig. 2A, E2 was found to inhibit mRNA levels of hepcidin in HuH7 cells by about 50%. Figure 2B shows that hepcidin mRNA levels were significantly inhibited by 30% after 24 h of treatment with E2 in HepG2 cells. As expected, the positive control IL-6 at 10 ng/ml significantly induced hepcidin mRNA levels in both HuH7 and HepG2 cells. This E2-mediated inhibitory effect was effectively abolished by pretreatment of a pure steroidal antiestrogen, ICI 182780 (Fig. 2C). ICI 182780 alone increased mRNA expression of hepcidin with or without E2 treatment. Because the antiestrogen activity of ICI 182780 is not caused by its ability to competitively antagonize E2 binding to the hormone binding site, but possibly by their ability to induce ER-dependent transcription (26), these results suggest that ER may have inhibitory effects on hepcidin without its ligand E2.
Fig. 2.
Inhibition of hepcidin mRNA expression by E2 in human liver HuH7 and HepG2 cells. A, HuH7 cells were treated with 10 and 100 nm E2 and 10 ng/ml IL-6 and mRNA levels of hepcidin were measured by RT-PCR. One representative gel from three independent experiments was shown. B, HepG2 cells were treated with E2 and hepcidin mRNA levels were measured by qPCR (n = 3). C, Inhibition of hepcidin mRNA by E2 and ICI 182780 pretreatment abolished E2-mediated hepcidin inhibition in HepG2 cells at 4- and 24-h treatments, respectively (n = 3). Y-axis was presented as a log scale. *, Significantly different from the untreated control.
Identification of functional ERE in the promoter region of hepcidin
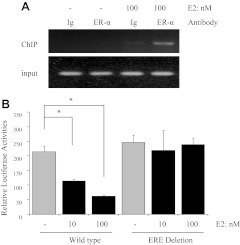
To identify which ERE responds to E2 treatment, ChIP assays with an ERα antibody were performed in HuH7 cells. Only one PCR product with a primer set between −2630 and −2072 was found after E2 treatment (Fig. 3A). All three other ERE (Fig. 1A) did not lead to the recruitment of ERα to the software-predicted sequences (data not shown). These results indicate that the ERE between −2630 and −2072 may be functional in E2-mediated hepcidin gene regulation. To further confirm that this ERE half-site is necessary for the response to E2 treatment in the hepcidin promoter, WT and the ERE half-site-deleted mutant, which were cloned into the luciferase reporter vector, were transiently transfected into HepG2 cells. Figure 3B shows that luciferase reporter activities were significantly decreased by E2 in a dose-dependent manner compared with the untreated control. More importantly, deletion of the ERE half-site completely abrogated the inhibition of hepcidin activity with E2 treatment.
Fig. 3.
Identification of functional ERE in the promoter region of hepcidin. A, Recruitment of ERα by E2 to the ERE site between −2630 and −2072 of the human hepcidin gene. After treating HuH7 cells with 100 nm E2 for 24 h, fragmented chromatin was immunoprecipitated with anti-ERα antibody and amplified by PCR using oligonucleotide primers flanking the ERE site. B, WT and the ERE half-site-deleted mutant were transfected into HepG2 cells. These cells were treated with E2 for 24 h and then analyzed for luciferase activity. The luciferase activity was normalized to Renilla luciferase activity, and the bar graph represents mean ± sd of three independent experiments. *, Significantly different from the untreated control.
Binding of ERE half-site to ERα
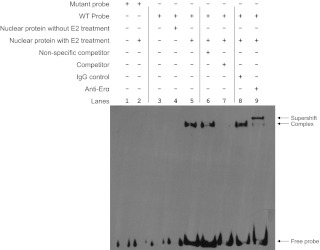
To determine whether the ERE half-site in the hepcidin gene can be recognized by ERα, HepG2 cells were treated with 100 nm E2. EMSA were performed using WT and mutant oligonucleotides harboring the ERE half-site of the hepcidin gene. Figure 4 shows efficient binding of nuclear proteins from E2-treated HepG2 cells to the WT oligonucleotide (lane 5) but not to the mutant oligonucleotide (lane 2). Nuclear extracts from untreated control cells did not show any bindings (lane 4). A nonspecific unlabeled competitor did not result in any changes in binding (lane 6). However, the binding was competed by adding 200-fold excess of unlabeled ERE probe (lane 7). More importantly, anti-ERα antibody (lane 9), but not normal IgG (lane 8), caused a supershift of the binding complex. Collectively, these data show that the ERE half-site of the hepcidin gene behaves similarly as the consensus ERE and that it can bind ERα.
Fig. 4.
Binding of nuclear proteins to the ERE of the hepcidin gene by EMSA. HepG2 cells were treated with or without 100 nm E2 for 24 h, and nuclear proteins were isolated and incubated with biotin-labeled mutant and WT oligonucleotides containing the ERE half-site of the hepcidin gene as described in Table 1. The resulting complexes were resolved by nondenaturing polyacrylamide gel electrophoresis. For competition EMSA, a 200-fold excess of unlabeled nonspecific or specific oligonucleotides harboring the ERE half-site of the hepcidin gene was added during the preincubation period. For supershift EMSA, anti-ERα antibody or normal human IgG was added. The arrows indicate supershift, ERE binding complex, and free probe.
In vivo inhibition of liver hepcidin mRNA by E2
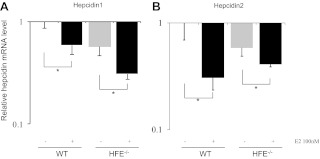
To further demonstrate the inhibitory effects of E2 on hepcidin promoter activity in vivo, WT and Hfe transgenic mice were ip injected E2. Blood, liver, and bone marrow samples were collected after 24 h of treatment. Figure 5A shows that mRNA levels of hepcidin-1 were significantly inhibited by E2 in WT mice. Hepcidin deficiency has been reported in HFE mutation carriers (27). We found that in Hfe knockout mice, background hepcidin mRNA levels were lower than those of WT mice. Interestingly, hepcidin mRNA was still inhibited by E2 in the Hfe knockout mice. Similarly, mRNA levels of hepcidin-2 were significantly decreased by E2 in both WT and Hfe mice.
Fig. 5.
Inhibition of hepcidin transcription in vivo by E2. WT and Hfe gene knockout mice were ip injected with 100 nmol E2/kg bodyweight (six mice per group). After killing at 24 h, liver samples were isolated and used for RNA extractions. Levels of mouse hepcidin-1 (A) and hepcidin-2 (B) mRNA were measured by qPCR. Data were presented as means ± sd. Y-axis was presented as a log scale. *, Significantly different from the untreated controls.
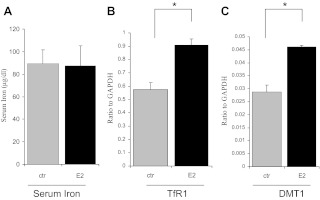
To relay this hepcidin inhibition to body iron homeostasis, Fig. 6A shows that there were no significant differences in serum iron between control and mice 24 h after receiving E2. However, mRNA levels of TfR1, a membrane receptor controlling cellular iron uptake, were significantly increased by E2 (Fig. 6B). Despite lower mRNA levels of DMT-1 than those of TfR1, DMT-1 mRNA was significantly increased by E2 as well (Fig. 6C). These results indicate an iron deficient status in bone marrow.
Fig. 6.
Alteration of body iron homeostasis in vivo by E2. WT mice (n = 6) were treated with E2 as described in the legend of Fig. 5. After killing at 24 h, blood samples were collected for measurements of serum iron (A) by ferrozine assay. Bone marrow samples were isolated from tibia and femora and used for qPCR analyses of TfR1 (B) and DMT-1 (C). Data were presented as means ± sd *, Significantly different from the untreated controls (ctr).
Discussion
Hepcidin is the central regulatory molecule of systemic iron homeostasis (5). In this study, we found that hepcidin mRNA is inhibited in cells and in mice by nanomolar concentrations of E2 (Figs. 2 and 5), which is physiologically or pharmacologically relevant to young women at the preovulatory phase or those taking contraceptives (see GPnotebook Wikipedia article on estradiol and Ref. 28). This inhibition is through a functional ERE half-site in the hepcidin promoter (Figs. 1, 3, and 4).
The effects of E2 on cellular iron homeostasis have been known to increase cellular iron uptake. E2 has been shown to enhance the expression of TfR1, in ER+ cells (29). E2 also induced a novel transferrin binding protein and lactoferrin in the female reproductive tract of mouse, rat, and hamster (30, 31), as well as the ferroxidase ceruloplasmin, the iron delivery protein lipocalin 2, and the iron-exporter ferroportin in the mouse uterus (32). E2-mediated transferrin gene expression in MCF-7 cells was shown through a nonconsensus distal ERE (33).
At the systemic level, an interaction of estrogen with iron has long been suspected. This is exemplified by a concurrent but inverse change in estrogen and iron levels during menopausal transition, with high estrogen and low iron in young women but low estrogen and high iron in older postmenopausal women (4). Furthermore, a time-dependent increase in iron stores has been shown in oral contraceptive users when compared with nonusers (34–36). The increased iron stores are said to be attributable primarily to reduced volume in menstrual blood loss. However, this cannot explain the animal data showing a significant rise in body iron stores and uptake in estrogen-treated ovariectomized rats, because rats do not have menses (37, 38). Clearly, there is a direct link between estrogen and systemic iron metabolism, although the molecular mechanism remains unclear.
In the present study, we showed that estrogen inhibited hepcidin expression through an ERE half-site in the promoter region of the hepcidin gene. Consistent with our finding, it has been shown that E2 reduced constitutive expression of hepcidin in fish (39). Like estrogen, a recent report shows that high levels of testosterone markedly suppressed serum hepcidin in men (40). Although we did not observe an increase in serum iron within 24 h of E2 injection (Fig. 6A), this may be due to two factors: 1) food intake significantly affects serum iron levels (16) (mice in our study were allowed food and water ad libitum and, thus, diminished our chance to detect a significant difference); and 2) others have shown that injection of E2 into rats does result in increased serum iron levels but several weeks after E2 injection (37, 38, 41). Thus, 24 h after E2 injection may be too short to allow us to observe an iron increase. Moreover, this slow rise in serum iron could be a result of hepcidin down-regulation by E2, an effect similar to anemia and hypoxia (14). A fast response in serum iron within hours was only reported by IL-6 and bone morphogenetic proteins, which up-regulate hepcidin (42). In addition, the slow response may be caused by the existence of a positive response element, which could delay the response time by the negative response element as identified here. All these factors on E2-mediated body iron homeostasis may be important and await further investigation.
Hepcidin controls serum iron concentration and tissue iron distribution by inhibiting intestinal iron absorption and preventing iron from recycling and mobilization of various tissues through ferroportin (12). The short-term effect of hepcidin changes could lead to an uneven iron distribution between serum and tissues (41, 43). High hepatic hepcidin mRNA was shown to be associated with an increase in splenic iron but a decrease in serum iron (43). In the present study, we expected that low hepcidin levels induced by E2 would lead to high ferroportin activity, resulting in an increased serum iron by increasing uptake from the guts but a deficiency in tissue iron through enhanced excretion. However, E2 did not alter serum iron but induced a low iron status in bone marrow, an important target of iron controlled by the hepcidin-ferroportin axis (44, 45). This was reflected by high levels of TfR1 and DMT-1 mRNA (Fig. 6). In contrast to our approach by injecting estrogen into the mice, it has been shown that estrogen withdrawal in female rats caused a significant decrease in serum iron but an increased iron in adipose tissues several weeks after ovariectomy surgery (41). These results suggest that estrogen deficiency up-regulates hepcidin, which inhibits intestinal iron absorption, leading to lower serum iron levels, as well as prevents iron release from adipocytes by increasing iron levels in the local adipose. These results are consistent with our finding of an inhibitory effect of estrogen on hepcidin regulation in WT as well as in Hfe transgenic mice. Hemochromatosis is an inherited iron overload disease that is caused by mutations in the HFE gene. Lowered hepcidin over long periods is an important contributor of iron overload in the HFE mutation carriers (46). We have shown that Hfe mutation leads to lower levels of hepcidin in Hfe transgenic mice compared with WT mice, and E2 further down-regulates hepcidin in the Hfe transgenic mice (Fig. 5). Because hepcidin has been shown to reverse iron overload in Hfe transgenic mice (47), our results suggest that down-regulation of hepcidin by E2 could increase iron levels not only in the WT mice but also in Hfe transgenic mice.
In conclusion, our results suggest that E2 could increase iron absorption and iron stores at the systemic levels during the proliferative phase (ovulation) to compensate iron losses, which occur during the menstruation phase in premenopausal women. Our present study also provides a novel mechanism of increased iron stores through direct inhibition of hepcidin by estrogens in contraceptive users. On the other hand, our results imply that increased iron observed in postmenopausal women is not a downstream effect of decreased estrogens during and after menopause (Supplemental Fig. 1).
Supplementary Material
Acknowledgments
Q.Y. performed the studies with the assistance of J.J. S.K. and S.B.A. aided in design of the experiments. X.H. conceived the experimental approach and provided overall supervision.
This work was supported in part by a seed grant from the New York University Musculoskeletal Center of Excellence and by the National Institutes of Health Grant R21 CA132684.
Disclosure Summary: The authors have nothing to disclose.
For editorial see page 2942
- AP-1
- Activator protein 1
- ChIP
- chromatin immunoprecipitation
- DMT-1
- divalent metal transporter-1
- E2
- 17β-estradiol
- ER
- estrogen receptor
- ERE
- estrogen responsive element
- GAPDH
- glyceraldehyde-3-phosphate dehydrogenase
- Hfe
- hemochromatosis Fe
- PTTG
- pituitary tumor transforming gene
- qPCR
- quantitative real-time PCR
- SP-1
- specificity protein-1
- TfR1
- transferrin receptor-1
- WT
- wild type.
References
- 1. Imai Y, Kondoh S, Kouzmenko A, Kato S. 2010. Minireview: osteoprotective action of estrogens is mediated by osteoclastic estrogen receptor-α. Mol Endocrinol 24:877–885 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Clemons M, Goss P. 2001. Estrogen and the risk of breast cancer. N Engl J Med 344:276–285 [DOI] [PubMed] [Google Scholar]
- 3. Huang X. 2008. Does iron have a role in breast cancer? Lancet Oncol 9:803–807 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Jian J, Pelle E, Huang X. 2009. Iron and menopause: does increased iron affect the health of postmenopausal women? Antioxid Redox Signal 11:2939–2943 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Hentze MW, Muckenthaler MU, Galy B, Camaschella C. 2010. Two to tango: regulation of mammalian iron metabolism. Cell 142:24–38 [DOI] [PubMed] [Google Scholar]
- 6. Zimmermann MB, Hurrell RF. 2007. Nutritional iron deficiency. Lancet 370:511–520 [DOI] [PubMed] [Google Scholar]
- 7. Eckard J, Dai J, Wu J, Jian J, Yang Q, Chen H, Costa M, Frenkel K, Huang X. 2010. Effects of cellular iron deficiency on the formation of vascular endothelial growth factor and angiogenesis. Iron deficiency and angiogenesis. Cancer Cell Int 10:28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Axelrod D, Smith J, Kornreich D, Grinstead E, Singh B, Cangiarella J, Guth AA. 2008. Breast cancer in young women. J Am Coll Surg 206:1193–1203 [DOI] [PubMed] [Google Scholar]
- 9. Gabriel CA, Domchek SM. 2010. Breast cancer in young women. Breast Cancer Res 12:212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Yang Q, Jian J, Abramson SB, Huang X. 2011. Inhibitory effects of iron on bone morphogenetic protein 2-induced osteoblastogenesis. J Bone Miner Res 26:1188–1196 [DOI] [PubMed] [Google Scholar]
- 11. Viatte L, Vaulont S. 2009. Hepcidin, the iron watcher. Biochimie 91:1223–1228 [DOI] [PubMed] [Google Scholar]
- 12. Nemeth E, Ganz T. 2006. Regulation of iron metabolism by hepcidin. Annu Rev Nutr 26:323–342 [DOI] [PubMed] [Google Scholar]
- 13. Bekri S, Gual P, Anty R, Luciani N, Dahman M, Ramesh B, Iannelli A, Staccini-Myx A, Casanova D, Ben Amor I, Saint-Paul MC, Huet PM, Sadoul JL, Gugenheim J, Srai SK, Tran A, Le Marchand-Brustel Y. 2006. Increased adipose tissue expression of hepcidin in severe obesity is independent from diabetes and NASH. Gastroenterology 131:788–796 [DOI] [PubMed] [Google Scholar]
- 14. Ganz T. 2011. Hepcidin and iron regulation, 10 years later. Blood 117:4425–4433 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Truksa J, Lee P, Peng H, Flanagan J, Beutler E. 2007. The distal location of the iron responsive region of the hepcidin promoter. Blood 110:3436–3437 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Zeleniuch-Jacquotte A, Zhang Q, Dai J, Shore RE, Arslan AA, Koenig KL, Karkoszka J, Afanasyeva Y, Frenkel K, Toniolo P, Huang X. 2007. Reliability of serum assays of iron status in postmenopausal women. Ann Epidemiol 17:354–358 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Klinge CM. 2001. Estrogen receptor interaction with estrogen response elements. Nucleic Acids Res 29:2905–2919 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Weisz A, Rosales R. 1990. Identification of an estrogen response element upstream of the human c-fos gene that binds the estrogen receptor and the AP-1 transcription factor. Nucleic Acids Res 18:5097–5106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Watkins RJ, Read ML, Smith VE, Sharma N, Reynolds GM, Buckley L, Doig C, Campbell MJ, Lewy G, Eggo MC, Loubiere LS, Franklyn JA, Boelaert K, McCabe CJ. 2010. Pituitary tumor transforming gene binding factor: a new gene in breast cancer. Cancer Res 70:3739–3749 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Rishi AK, Shao ZM, Baumann RG, Li XS, Sheikh MS, Kimura S, Bashirelahi N, Fontana JA. 1995. Estradiol regulation of the human retinoic acid receptor α gene in human breast carcinoma cells is mediated via an imperfect half-palindromic estrogen response element and Sp1 motifs. Cancer Res 55:4999–5006 [PubMed] [Google Scholar]
- 21. Mason CE, Shu FJ, Wang C, Session RM, Kallen RG, Sidell N, Yu T, Liu MH, Cheung E, Kallen CB. 2010. Location analysis for the estrogen receptor-α reveals binding to diverse ERE sequences and widespread binding within repetitive DNA elements. Nucleic Acids Res 38:2355–2368 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Das D, Peterson RC, Scovell WM. 2004. High mobility group B proteins facilitate strong estrogen receptor binding to classical and half-site estrogen response elements and relax binding selectivity. Mol Endocrinol 18:2616–2632 [DOI] [PubMed] [Google Scholar]
- 23. Martini PG, Katzenellenbogen BS. 2001. Regulation of prothymosin α gene expression by estrogen in estrogen receptor-containing breast cancer cells via upstream half-palindromic estrogen response element motifs. Endocrinology 142:3493–3501 [DOI] [PubMed] [Google Scholar]
- 24. DeNardo DG, Kim HT, Hilsenbeck S, Cuba V, Tsimelzon A, Brown PH. 2005. Global gene expression analysis of estrogen receptor transcription factor cross talk in breast cancer: identification of estrogen-induced/activator protein-1-dependent genes. Mol Endocrinol 19:362–378 [DOI] [PubMed] [Google Scholar]
- 25. Nemeth E, Rivera S, Gabayan V, Keller C, Taudorf S, Pedersen BK, Ganz T. 2004. IL-6 mediates hypoferremia of inflammation by inducing the synthesis of the iron regulatory hormone hepcidin. J Clin Invest 113:1271–1276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Dudley MW, Sheeler CQ, Wang H, Khan S. 2000. Activation of the human estrogen receptor by the antiestrogens ICI 182,780 and tamoxifen in yeast genetic systems: implications for their mechanism of action. Proc Natl Acad Sci USA 97:3696–3701 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Papanikolaou G, Tzilianos M, Christakis JI, Bogdanos D, Tsimirika K, MacFarlane J, Goldberg YP, Sakellaropoulos N, Ganz T, Nemeth E. 2005. Hepcidin in iron overload disorders. Blood 105:4103–4105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Schiavon R, Benavides S, Oropeza G, Garza-Flores J, Recio R, Díaz-Sanchez V, Pérez-Palacios G. 1988. Serum estrogens and ovulation return in chronic users of a once-a-month injectable contraceptive. Contraception 37:591–598 [DOI] [PubMed] [Google Scholar]
- 29. Dai J, Jian J, Bosland M, Frenkel K, Bernhardt G, Huang X. 2008. Roles of hormone replacement therapy and iron in proliferation of breast epithelial cells with different estrogen and progesterone receptor status. Breast 17:172–179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Poola I, Kiang JG. 1994. The estrogen-inducible transferrin receptor-like membrane glycoprotein is related to stress-regulated proteins. J Biol Chem 269:21762–21769 [PubMed] [Google Scholar]
- 31. Teng CT, Beard C, Gladwell W. 2002. Differential expression and estrogen response of lactoferrin gene in the female reproductive tract of mouse, rat, and hamster. Biol Reprod 67:1439–1449 [DOI] [PubMed] [Google Scholar]
- 32. Stuckey R, Aldridge T, Lim FL, Moore DJ, Tinwell H, Doherty N, Davies R, Smith AG, Kimber I, Ashby J, Orphanides G, Moggs JG. 2006. Induction of iron homeostasis genes during estrogen-induced uterine growth and differentiation. Mol Cell Endocrinol 253:22–29 [DOI] [PubMed] [Google Scholar]
- 33. Vyhlidal C, Li X, Safe S. 2002. Estrogen regulation of transferrin gene expression in MCF-7 human breast cancer cells. J Mol Endocrinol 29:305–317 [DOI] [PubMed] [Google Scholar]
- 34. Casabellata G, Di Santolo M, Banfi G, Stel G, Gonano F, Cauci S. 2007. Evaluation of iron deficiency in young women in relation to oral contraceptive use. Contraception 76:200–207 [DOI] [PubMed] [Google Scholar]
- 35. Frassinelli-Gunderson EP, Margen S, Brown JR. 1985. Iron stores in users of oral contraceptive agents. Am J Clin Nutr 41:703–712 [DOI] [PubMed] [Google Scholar]
- 36. Milman N, Kirchhoff M. 1992. Iron stores in 1359, 30- to 60-year-old Danish women: evaluation by serum ferritin and hemoglobin. Ann Hematol 64:22–27 [DOI] [PubMed] [Google Scholar]
- 37. Borràs M. 1998. Hormone dependency of splenic iron stores in the rat: effect of oestrogens on the recuperation of reserves in ferrodeficient subjects. Lab Anim 32:290–297 [DOI] [PubMed] [Google Scholar]
- 38. Haouari M, Haouari-Oukerro F, Alguemi C, Nagati K, Zouaghi H, Kamoun A. 1994. Effects of oestradiol-17β on small intestine iron absorption and iron uptake into blood and liver. Horm Metab Res 26:53–54 [DOI] [PubMed] [Google Scholar]
- 39. Robertson LS, Iwanowicz LR, Marranca JM. 2009. Identification of centrarchid hepcidins and evidence that 17β-estradiol disrupts constitutive expression of hepcidin-1 and inducible expression of hepcidin-2 in largemouth bass (Micropterus salmoides). Fish Shellfish Immunol 26:898–907 [DOI] [PubMed] [Google Scholar]
- 40. Bachman E, Feng R, Travison T, Li M, Olbina G, Ostland V, Ulloor J, Zhang A, Basaria S, Ganz T, Westerman M, Bhasin S. 2010. Testosterone suppresses hepcidin in men: a potential mechanism for testosterone-induced erythrocytosis. J Clin Endocrinol Metab 95:4743–4747 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Mattace Raso G, Irace C, Esposito E, Maffettone C, Iacono A, Di Pascale A, Santamaria R, Colonna A, Meli R. 2009. Ovariectomy and estrogen treatment modulate iron metabolism in rat adipose tissue. Biochem Pharmacol 78:1001–1007 [DOI] [PubMed] [Google Scholar]
- 42. Kemna E, Pickkers P, Nemeth E, van der Hoeven H, Swinkels D. 2005. Time-course analysis of hepcidin, serum iron, and plasma cytokine levels in humans injected with LPS. Blood 106:1864–1866 [DOI] [PubMed] [Google Scholar]
- 43. Camberlein E, Abgueguen E, Fatih N, Canonne-Hergaux F, Leroyer P, Turlin B, Ropert M, Brissot P, Loréal O. 2010. Hepcidin induction limits mobilisation of splenic iron in a mouse model of secondary iron overload. Biochim Biophys Acta 1802:339–346 [DOI] [PubMed] [Google Scholar]
- 44. Andrews NC. 1999. Disorders of iron metabolism. N Engl J Med 341:1986–1995 [DOI] [PubMed] [Google Scholar]
- 45. Masson C. 2011. Rheumatoid anemia. Joint Bone Spine 78:131–137 [DOI] [PubMed] [Google Scholar]
- 46. Gao J, Chen J, De Domenico I, Koeller DM, Harding CO, Fleming RE, Koeberl DD, Enns CA. 2010. Hepatocyte-targeted HFE and TFR2 control hepcidin expression in mice. Blood 115:3374–3381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Morán-Jiménez MJ, Méndez M, Santiago B, Rodríguez-García ME, Moreno-Carralero MI, Sánchez-Lucío AC, Grau M, Enríquez-de-Salamanca R. 2010. Hepcidin treatment in Hfe−/− mice diminishes plasma iron without affecting erythropoiesis. Eur J Clin Invest 40:511–517 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.