Abstract
AIM: To evaluate the feasibility, safety and efficacy of ultrasound-guided microwave (MW) ablation for abdominal wall metastatic tumors.
METHODS: From August 2007 to December 2010, a total of 11 patients with 23 abdominal wall nodules (diameter 2.59 cm ± 1.11 cm, range 1.3 cm to 5.0 cm) were treated with MW ablation. One antenna was inserted into the center of tumors less than 1.7 cm, and multiple antennae were inserted simultaneously into tumors 1.7 cm or larger. A 21 gauge thermocouple was inserted near important organs which required protection (such as bowel or gallbladder) for real-time temperature monitoring during MW ablation. Treatment outcome was observed by contrast-enhanced ultrasound and magnetic resonance imaging (MRI) [or computed tomography (CT)] during follow-up.
RESULTS: MW ablation was well tolerated by all patients. Six patients with 11 nodules had 1 thermocouple inserted near important organs for real-time temperature monitoring and the maximum temperature was 56 °C. Major complications included mild pain (54.5%), post-ablation fever (100%) and abdominal wall edema (25%). All 23 tumors (100%) in this group were completely ablated, and no residual tumor or local recurrence was observed at a median follow-up of 13 mo (range 1 to 32 mo). The ablation zone was well defined on contrast-enhanced imaging (contrast-enhanced CT, MRI and/or contrast-enhanced ultrasound) and gradually shrank with time.
CONCLUSION: Ultrasound-guided MW ablation may be a feasible, safe and effective treatment for abdominal wall metastatic tumors in selected patients.
Keywords: Abdominal wall, Microwave ablation, Neoplasm metastasis, Thermal ablation therapy, Ultrasonography
INTRODUCTION
Clinically, the incidence of primary abdominal wall malignant tumors is low. Usually metastasis or local infiltration is the major cause of abdominal wall metastatic tumors. A number of abdominal wall tumors occur during or after therapy, and are difficult to cure. Currently, most studies report that the main treatment for abdominal wall tumors is resection[1], however some patients are unable to undergo resection due to tumor stage. Small subcutaneous lesions can be easily resected, whereas it is technically difficult for radical excision of large lesions, especially those which invade muscles. Moreover, surgical reconstruction is also troublesome, and a significant number of patients require abdominoplasty for larger abdominal wall tumors[2-4]. Other treatments have been used for abdominal wall tumors, such as radiotherapy, chemotherapy and thermal ablation. Radiotherapy requires patients to have optimal health status, while chemotherapy often plays an additional role and is not used as a radical cure. Thermal ablation is a minimally invasive technique, and has been widely used for the treatment of primary and metastatic liver cancer in past decades and is well established[5-8]. High intensity focused ultrasound (HIFU) and radiofrequency ablation have been used in abdominal wall metastatic tumors with curative effect[9]. Microwave (MW) ablation for the treatment of liver tumorsis relatively low-risk and has favorable therapeutic efficacy[10,11]. Compared with radiofrequency ablation, MW energy does not appear to be limited by charring and tissue desiccation, thus, thermal efficiency may be considerably higher with MW systems than with radiofrequency systems[12-15]. To our knowledge, there are no reports assessing the efficacy and safety of MW ablation for abdominal wall tumors under ultrasound guidance.
Thus, the purpose of this study was to assess the effectiveness of MW ablation for abdominal wall tumors under ultrasound guidance in the short and medium term, and to identify the possible complications that determine the rate of therapeutic success.
MATERIALS AND METHODS
Patients
From August 2007 to December 2010, eleven patients with 23 abdominal wall tumors were enrolled in this retrospective study (Table 1). The patients were 6 men and 5 women aged 35-68 years (mean age, 54.18 ± 9.14 years), and tumor size ranged from 1.3 cm to 5.0 cm in maximum diameter (mean diameter 2.59 ± 1.11 cm, range 1.3 cm to 5.0 cm). Before MW ablation, 2 patients were treated with chemotherapy, 1 patient was treated with chemotherapy and radiotherapy, and 1 patient with immunotherapy. Informed consent was obtained from all patients at enrollment. The inclusion criteria for this study were as follows: (1) The entire tumor could be clearly seen on ultrasound; (2) The tumor size was no more than 5 cm in diameter; (3) The tumor was located more than 5 mm from the skin surface; (4) The tumor had not adhered to the peritoneum or bowels; and (5) The tumor had not invaded the bones. The exclusion criteria were as follows: (1) Severe cardiopulmonary disease; (2) Severe coagulation abnormalities (prothrombin time more than 25 s, prothrombin activity higher than 40%, and platelet count higher than 40 cells × 109/L); and (3) Infection. All selected patients chose MW ablation on the basis of tumor stage which made them inoperable, or had comorbidities, advanced age, or refused to undergo surgery. All abdominal wall nodules were metastatic lesions. In five cases abdominal wall tumors had metastasized from hepatocellular carcinoma (HCC); two cases metastasized from adrenal glands, whose pathological types were adrenal cortical carcinoma and pheochromocytoma. The remaining four cases metastasized from lung, ovary, bladder and kidney; and the corresponding pathological types were lung adenocarcinoma, ovarian peritoneal serous papillary carcinoma, bladder transitional cell carcinoma and renal clear cell carcinoma. The primary liver lesions in five patients were treated with MW ablation, and the primary lesions in the remaining patients were resected. Abdominal wall metastatic tumors in three patients with HCC were caused by needle tract seeding, which appeared 9, 11 and 22 mo after liver puncture.
Table 1.
Patient and tumor characteristics
| No. | Age (yr) | Sex | Tumor type | Tumor number | Tumor size (cm) | Antenna | Antenna number | Ablation power (W) | Ablation time (min) | Session | Follow up (mo) |
| 1 | 59 | M | HCC | 1 | 1.8 | T11 | 1 | 50 | 6 | 1 | 31 |
| 2 | 35 | M | HCC | 1 | 2.1 | T11 | 1 | 50 | 8 | 1 | 26 |
| 3 | 68 | M | HCC | 2 | 4.3 | T11 | 2 | 40 | 5 | 2 | 19 |
| 4.8 | T11 | 2 | 50 | 16.5 | 1 | 19 | |||||
| 4 | 57 | F | Ovary serous papillary adenocarcinoma | 1 | 2.2 | T7/T11 | 2 | 45 | 2 | 2 | 18 |
| 5 | 55 | F | Lung adenocarcinomas | 2 | 1.3 | T11 | 1 | 50 | 3.5 | 1 | 18 |
| 1.7 | T11 | 1 | 50 | 3.5 | 1 | 18 | |||||
| 6 | 56 | F | Adrenocortical carcinoma | 4 | 4 | T11 | 2 | 60 | 12 | 1 | 9 |
| 3.1 | T11 | 2 | 30 | 12 | 1 | 9 | |||||
| 3.3 | T7/T11 | 2 | 30 | 16 | 1 | 9 | |||||
| 2.5 | T5 | 2 | 50 | 5 | 1 | 9 | |||||
| 7 | 58 | M | HCC | 1 | 1.3 | T11 | 2 | 50 | 5 | 1 | 3 |
| 8 | 48 | M | Bladder adenocarcinoma | 2 | 5 | T11 | 2 | 40 | 15 | 2 | 13 |
| 2.1 | T11 | 2 | 50 | 11.8 | 1 | 13 | |||||
| 9 | 59 | F | Renal clear cell carcinoma | 5 | 2.2 | T11 | 2 | 60 | 5 | 1 | 15 |
| 2.1 | T11 | 2 | 50 | 5.5 | 1 | 15 | |||||
| 2.1 | T11 | 2 | 50 | 6 | 1 | 15 | |||||
| 2 | T7/T11 | 2 | 45 | 4 | 1 | 15 | |||||
| 1.6 | T11 | 1 | 45 | 3.8 | 1 | 15 | |||||
| 10 | 58 | F | HCC | 1 | 2.6 | T5 | 1 | 50 | 13 | 1 | 1 |
| 11 | 42 | M | Adrenal pheochromocytoma | 3 | 3.1 | T7 | 1 | 50 | 8 | 1 | 4 |
| 2.8 | T5 | 2 | 50 | 6.5 | 1 | 4 | |||||
| 1.4 | T5 | 1 | 50 | 1.2 | 1 | 4 |
M: Male; F: Female; HCC: Hepatocellular carcinoma.
Equipment
A KY2000 MW ablation system (Kangyou Medical Instruments, Nanjing, China) consisting of two independent MW generators, two flexible coaxial cables and two water-pumping machines, which could drive two 15-gauge cooled-shaft antennae simultaneously was used. The generators are capable of producing 1-100 W of power at 2450 MHz. The cooled shaft antenna was coated with polytetrafluoroethylene to prevent adhesion, which can also be clearly seen on ultrasound. The antenna is designed to minimize power feedback and provide tissue with optimal energy deposition. Three types of antennae were applied according to the size and location of the tumor, the antennae tips were 0.5, 0.7 and 1.1 cm, respectively. For tumors smaller than 2 cm, an antenna tip of 0.5 cm was chosen, while for tumors larger than 3 cm, a tip of 1.1 cm was selected. The MW machine is also equipped with a thermal monitoring system with 21-gauge thermocouple needles, which can be placed percutaneously at a designated location to monitor the temperature during real-time ablation.
Ablation procedures
Before treatment, all patients were scanned using contrast-enhanced computed tomography (CT)/magnetic resonance imaging (MRI) and ultrasound, and an appropriate puncture route was chosen by ultrasound. After local anesthesia with 1% lidocaine, the antenna was inserted percutaneously into the tumor and placed at designated sites under ultrasound guidance. Histologic diagnoses were confirmed by guided sonography with an 18-gaugecutting-edge needle through an automated biopsy gun device before inserting the antenna, and specimens were taken from different parts of the tumor (one to three pieces). One antenna was inserted into the center of tumors less than 1.7 cm, and multiple antennae were inserted into tumors 1.7 cm or larger. General anesthesia (Propofol, 6-12 mg/kg per hour; Ketamine, 1-2 mg/kg) was applied after correct placement of antennae, and MWs were then emitted[16,17]. Two antennae were used simultaneously during MW ablation to achieve a larger ablation zone. If the tumor was adjacent to bowel, gallbladder or other important tissues, a 21 gauge thermocouple was inserted close to these tissues for real-time temperature monitoring during MW ablation. The treatment session ended if the transient hyperechoic zone between the antennae merged and covered the target region on gray-scale ultrasound. Simultaneously, according to our previous clinical experience, the temperature of the thermal needles should not exceed the target temperature to avoid heat injury in these organs[18]. For tumors with subcutaneous invasion, an ice bag was placed on the skin to avoid scalding during MW ablation.
Postprocedural observation and follow-up
After MW ablation, patients were closely monitored for possible complications such as fever, skin burns and pain which were also documented. All patients underwent contrast-enhanced ultrasound 3 d after MW ablation to assess treatment efficacy. If residual tumor (hyper-enhanced area on contrast-enhanced ultrasound) was found, a further session was planned or patients entered the follow-up protocol, which consisted of contrast-enhanced CT, MRI and/or contrast-enhanced ultrasound 1, 3 and 6 mo after MW ablation, and every 6 mo thereafter. Enhanced areas on the abdominal wall were assumed to represent viable tumors. If residual or recurrent tumor on the abdominal or chest wall was detected, a further MW ablation session was planned if the lesion still met the inclusion criteria.
RESULTS
MW ablation was well tolerated by all patients. The output power ranged from 30 W to 50 W, an output setting of 50 W was routinely used during ablation sessions, relatively lower than that used in liver lesions. Four of 23 tumors were adjacent to the intestinal tract, one nodule was adjacent to the gallbladder, and 1 nodule was adjacent to the gallbladder and intestinal tract. A thermocouple was inserted adjacent to these high-risk locations for real-time temperature monitoring. In this study, we used 1 thermocouple with a maximum temperature of 56 °C in 6 patients with 11 nodules. The treatment time was no more than 16.5 min (mean time 7.6 ± 4.3 min, range 2 min to 16.5 min). Twenty-one of 23 tumors were successfully eradicated following one MW session. The other two tumors underwent two MW sessions; one tumor was near the adrenal gland, and the other was a tumor 5 cm in maximum diameter. All 23 tumors (100%) in this group were completely ablated which was confirmed by follow-up imaging during a period of 1-32 mo. No major complications were encountered after MW ablation. Six patients experienced grade 1 according to standardization of terms and reporting criteria for image-guided tumor ablation[19]. Post-ablation fever was encountered in all patients, but each patient’s temperature was lower than 38 °C and no drugs were needed. No skin burns were observed in the treated area, however, the treated area was slightly swollen in 3 patients. The patients were followed up until January 20, 2011. The median survival period of the 11 patients after MW ablation was 15.0 mo. During a mean follow-up of 13 mo (range 1-32 mo), three patients died of primary tumor progression, however, the treated tumors were unenhanced on follow-up contrast-enhanced images. In the other 8 patients, the ablation zones were well defined on contrast-enhanced images, and gradually shrank with time (Figure 1). Two patients developed distant metastases, one patient was treated with repeated sessions of MW ablation, and the other underwent HIFU.
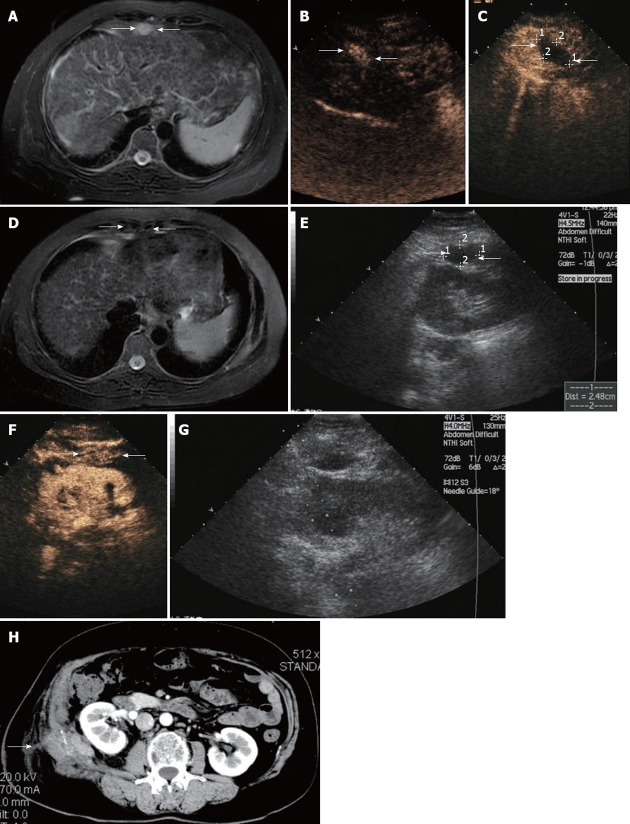
Figure 1.
Ultrasound findings in a 56-year-old woman with abdominal wall tumor metastasized from liver and adrenal gland cancer. A: Contrast-enhanced magnetic resonance imaging (MRI) scan shows a lesion with hyperenhancement in T2-weighted images in the abdominal wall (arrow); B: Arterial phase in contrast-enhanced ultrasound (CEUS) shows hyperenhancement within the lesions (arrow); C: CEUS scan obtained 3 d after microwave (MW) ablation shows a hypoechoic area with no enhancement, suggestive of complete necrosis (arrow); D: Contrast-enhanced MRI scan shows a lesion with hypoenhancement in T2-weighted phase MR image obtained 1 mo after MW ablation revealing complete ablation. Contrast-enhanced MRI scan shows a lesion with hypoenhancement in T2-weighted images in the abdominal wall (arrow); E: Sonogram obtained before MW ablation shows hypoechoic nodule of 2.48 cm in maximum diameter in the abdominal wall (arrow); F: Arterial phase in CEUS shows hyperenhancement within the lesions (arrow); G: Sonogram obtained during MW ablation shows one antenna being inserted into the nodule; H: Abdominal wall edema occurred at the right lumbar in the arterial phase of contrast-enhanced CT (arrow).
DISCUSSION
In the past few decades, the treatment of abdominal wall tumors, especially metastasis, has evolved[9,20]. In addition to traditional surgical resection, there are many other treatments, such as transarterial embolization[20] radiofrequency ablation and HIFU[21]. Surgical resection is the first choice for abdominal tumors, although it carries a risk of hemorrhage and possible post-operative incisional hernia, and patients usually require reconstruction, such as abdominoplasty[22]. Some patients may not be surgical candidates due to poor medical conditions[23]. It is difficult to create a safety margin to eradicate possible microscopic tumor foci using transarterial embolization of the feeding vessel of the abdominal tumor, and may cause ischemic changes. Thus, it is rarely used in abdominal tumors. Compared with surgical resection, HIFU is a less invasive alternative to surgical resection. However, due to the bio-effects of focused ultrasound during the procedure, heat diffusion out of the focal region is inevitable and can damage the surrounding tissues.
We have also made some progress with the antennae used in MW ablation.Three types of antennae were used in this study according to tumor size and location, and the antennae tips were 0.5, 0.7 and 1.1 cm, respectively. According to a preliminary study, an antenna tip of 1.1 cm can ablate 2-2.6 cm ex vivo porcine livers with the output power of 60 W and the setting time of 10 min[24]. Based on these preliminary experiments, an antenna tip of 0.7 cm and 0.5 cm can ablate 2.4-2.6 cm and 2.2-2.4 cm ex vivo porcine livers with the output power of 60 W and the setting time of 10 min, respectively. The new type tips are safer, because the ablation zone is relatively small which avoids burning the skin in the superficial tumor during the procedure. Therefore, percutaneous MW ablation may be clinically feasible for superficial tumors. Based on our previous experience in MW ablation for HCC, we performed MW ablation on abdominal tumors. Treatment efficacy was encouraging. For tumors smaller than 4 cm, radical cure was achieved in all nodules within no more than 12 min and no tumor recurrence was noted during follow-up. In order to achieve the same effect, tumors larger than 4 cm needed several sessions. MW ablation, is a relatively new technique and can be used in different types of tumors[25-30], the primary effectiveness rate is equal to HIFU and radiofrequency ablation[16,31]. The favorable effectiveness of MW ablation may be attributed to its potential advantages[32], such as larger volume of ablation zone, reduced treatment time, less influence on the perfusion-mediated heat sink effect, higher thermal efficiency and the possibility of placing multiple antennae simultaneously[33-35], especially compared with radiofrequency ablation. Results suggest that, like other techniques, MW ablation may be safe and effective for abdominal wall tumors, it may also represent a competitive alternative to surgical resection and other therapies. The high effectiveness rate of MW ablation may due to the following 4 reasons: (1) The new-type antenna used in this study was capable of ablating superficial tumors more securely; (2) There were relatively strict inclusion and exclusion criteria; (3) All operations were performed by experienced doctors (Yu XL and Liang P); and (4) Real-time temperature monitoring served as an indicator for predicting reliable safety margins. No severe complications were observed in this study. Unlike the treatment of liver tumors, abdominal tumor ablation has its own complication of abdominal wall edema. We studied three patients who had abdominal wall edema and found that the ablated tumors were all located in muscles and with subcutaneous invasion. Compared with parenchymal organs, muscle tissue lacks relative capsules and can not accumulate heat. During the ablation procedure, heat overflow in the muscle bundle can readily lead to abdominal wall edema. In order to ablate completely, the actual ablation zone should be larger than the size of the tumor, which will ablate normal tissues (such as fatty tissue or muscle tissue) and cause edema in a short time. Fortunately, the abdominal wall edema seen in three patients was very mild and all patients recovered within a short time (1-3 mo) without special treatment. For the ablation of specific tumors, such as pheochromocytoma, we used low power at the beginning of MW ablation, and changed the power according to the blood pressure. The use of antihypertensive drugs during the procedure should be taken into account, as pheochromocytomas can release catecholamine which could result in blood pressure fluctuation. There are key points during MW ablation which can reduce the incidence of complications: (1) The MW antenna was inserted in the deepest area of the tumor; (2) Increasing the angle of puncture between the antenna and transducer, that is insert the antenna along the long axis of the tumor for conformable ablation; and (3) For tumors with subcutaneous invasion, the transient hyperechoic zone did not exceed the dermal layer in the gray-scan ultrasound, and an ice bag was placed on the skin to avoid scalding. Although this was not a randomized, controlled study of traditional techniques, the low complication rate, minimal side effects, rapid recovery and lower costs (compared with radiofrequency ablation in China) strongly favor MW ablation as an optional curative approach for abdominal wall tumors. This study has some limitations: (1) Only 11 patients were included in this study. More patients should be recruited in order to assess the efficacy of this treatment; (2) Follow-up was relatively short and we are uncertain of the long-term results; and (3) The study did not include a comparison with other treatments.
In conclusion, our preliminary results showed that ultrasound-guided MW ablation appeared to be effective in the treatment of abdominal wall tumors. Further studies are warranted to observe its long-term efficacy and the results should be compared with other treatments.
COMMENTS
Background
A number of abdominal wall tumors occur during or after therapy, and are difficult to cure. Currently, the main treatment for abdominal wall tumors is resection, while the excision rate is low. Microwave (MW) ablation for the treatment of liver tumors has relatively low-risk and favorable therapeutic efficacy. However, there are no reports assessing the efficacy and safety of MW ablation for abdominal wall tumors under ultrasound guidance.
Research frontiers
MW ablation, as a relatively new technique, and can be applied to different types of tumors. This research was concerned with applying MW ablation in patients with abdominal wall metastatic tumors, and to improve the effectiveness rate of this technique.
Innovations and breakthroughs
Unlike the commonly used antenna, the authors made some progress with the antennae used in this study. Three types of antennae were used according to tumor size and location, and the antennae tips used were 0.5, 0.7 and 1.1 cm, respectively. These new type tips are safer, because the ablation zone is relatively small which avoids burning the skin in the superficial tumor during the procedure. Therefore, percutaneous MW ablation may be clinically feasible for superficial tumors.
Applications
The study results suggest that ultrasound-guided MW ablation may be a feasible, safe and effective treatment of abdominal wall metastatic tumors in selected patients.
Terminology
Image-guided tumor ablation: The term tumor ablation is defined as the direct application of chemical or thermal therapies to a specific focal tumor (or tumors) in an attempt to achieve eradication or substantial tumor destruction.
Peer review
The authors present short and medium-term outcomes for ultrasound-guided MW ablation for abdominal wall metastatic tumors. The procedure was well tolerated by all patients with the most significant complication being abdominal wall edema which resolved without treatment in all cases.
Footnotes
Peer reviewer: David A Iannitti, Professor, Department of General Surgery, Carolinas Medical Center, Charlotte, NC 28204, United States
S- Editor Gou SX L- Editor Webster JR E- Editor Li JY
References
- 1.Stojadinovic A, Hoos A, Karpoff HM, Leung DH, Antonescu CR, Brennan MF, Lewis JJ. Soft tissue tumors of the abdominal wall: analysis of disease patterns and treatment. Arch Surg. 2001;136:70–79. doi: 10.1001/archsurg.136.1.70. [DOI] [PubMed] [Google Scholar]
- 2.Lazzeri D, Pascone C, Agostini T. Abdominal wall reconstruction: some historical notes. Plast Reconstr Surg. 2010;126:1793–1794; author reply 1794. doi: 10.1097/PRS.0b013e3181ef9287. [DOI] [PubMed] [Google Scholar]
- 3.Gu Y, Tang R, Gong DQ, Qian YL. Reconstruction of the abdominal wall by using a combination of the human acellular dermal matrix implant and an interpositional omentum flap after extensive tumor resection in patients with abdominal wall neoplasm: a preliminary result. World J Gastroenterol. 2008;14:752–757. doi: 10.3748/wjg.14.752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yezhelyev MV, Deigni O, Losken A. Management of Full Thickness Abdominal Wall Defects Following Tumor Resection. Ann Plast Surg. 2011:May 27; Epub ahead of print. doi: 10.1097/SAP.0b013e31821d0715. [DOI] [PubMed] [Google Scholar]
- 5.Liu JG, Wang YJ, Du Z. Radiofrequency ablation in the treatment of small hepatocellular carcinoma: a meta analysis. World J Gastroenterol. 2010;16:3450–3456. doi: 10.3748/wjg.v16.i27.3450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Vanagas T, Gulbinas A, Pundzius J, Barauskas G. Radiofrequency ablation of liver tumors (II): clinical application and outcomes. Medicina (Kaunas) 2010;46:81–88. [PubMed] [Google Scholar]
- 7.Mayo SC, Pawlik TM. Thermal ablative therapies for secondary hepatic malignancies. Cancer J. 2010;16:111–117. doi: 10.1097/PPO.0b013e3181d7ea07. [DOI] [PubMed] [Google Scholar]
- 8.Liang P, Wang Y, Yu X, Dong B. Malignant liver tumors: treatment with percutaneous microwave ablation--complications among cohort of 1136 patients. Radiology. 2009;251:933–940. doi: 10.1148/radiol.2513081740. [DOI] [PubMed] [Google Scholar]
- 9.Wang Y, Wang W, Wang Y, Tang J. Ultrasound-guided high-intensity focused ultrasound treatment for needle-track seeding of hepatocellular carcinoma: preliminary results. Int J Hyperthermia. 2010;26:441–447. doi: 10.3109/02656731003705686. [DOI] [PubMed] [Google Scholar]
- 10.Liang P, Dong B, Yu X, Yu D, Wang Y, Feng L, Xiao Q. Prognostic factors for survival in patients with hepatocellular carcinoma after percutaneous microwave ablation. Radiology. 2005;235:299–307. doi: 10.1148/radiol.2351031944. [DOI] [PubMed] [Google Scholar]
- 11.Martin RC, Scoggins CR, McMasters KM. Safety and efficacy of microwave ablation of hepatic tumors: a prospective review of a 5-year experience. Ann Surg Oncol. 2010;17:171–178. doi: 10.1245/s10434-009-0686-z. [DOI] [PubMed] [Google Scholar]
- 12.Wright AS, Lee FT, Mahvi DM. Hepatic microwave ablation with multiple antennae results in synergistically larger zones of coagulation necrosis. Ann Surg Oncol. 2003;10:275–283. doi: 10.1245/aso.2003.03.045. [DOI] [PubMed] [Google Scholar]
- 13.Tanaka T, Westphal S, Isfort P, Braunschweig T, Penzkofer T, Bruners P, Kichikawa K, Schmitz-Rode T, Mahnken AH. Microwave Ablation Compared with Radiofrequency Ablation for Breast Tissue in an Ex Vivo Bovine Udder Model. Cardiovasc Intervent Radiol. 2011:Aug 11; Epub ahead of print. doi: 10.1007/s00270-011-0253-4. [DOI] [PubMed] [Google Scholar]
- 14.Simo KA, Sereika SE, Newton KN, Gerber DA. Laparoscopic-assisted microwave ablation for hepatocellular carcinoma: safety and efficacy in comparison with radiofrequency ablation. J Surg Oncol. 2011;104:822–829. doi: 10.1002/jso.21933. [DOI] [PubMed] [Google Scholar]
- 15.Yu J, Liang P, Yu X, Liu F, Chen L, Wang Y. A comparison of microwave ablation and bipolar radiofrequency ablation both with an internally cooled probe: results in ex vivo and in vivo porcine livers. Eur J Radiol. 2011;79:124–130. doi: 10.1016/j.ejrad.2009.12.009. [DOI] [PubMed] [Google Scholar]
- 16.Dong BW, Liang P, Yu XL, Zeng XQ, Wang PJ, Su L, Wang XD, Xin H, Li S. Sonographically guided microwave coagulation treatment of liver cancer: an experimental and clinical study. AJR Am J Roentgenol. 1998;171:449–454. doi: 10.2214/ajr.171.2.9694473. [DOI] [PubMed] [Google Scholar]
- 17.Dong B, Liang P, Yu X, Su L, Yu D, Cheng Z, Zhang J. Percutaneous sonographically guided microwave coagulation therapy for hepatocellular carcinoma: results in 234 patients. AJR Am J Roentgenol. 2003;180:1547–1555. doi: 10.2214/ajr.180.6.1801547. [DOI] [PubMed] [Google Scholar]
- 18.Zhou P, Liang P, Yu X, Wang Y, Dong B. Percutaneous microwave ablation of liver cancer adjacent to the gastrointestinal tract. J Gastrointest Surg. 2009;13:318–324. doi: 10.1007/s11605-008-0710-9. [DOI] [PubMed] [Google Scholar]
- 19.Goldberg SN, Charboneau JW, Dodd GD, Dupuy DE, Gervais DA, Gillams AR, Kane RA, Lee FT, Livraghi T, McGahan JP, et al. Image-guided tumor ablation: proposal for standardization of terms and reporting criteria. Radiology. 2003;228:335–345. doi: 10.1148/radiol.2282021787. [DOI] [PubMed] [Google Scholar]
- 20.Shibata T, Shibata T, Maetani Y, Kubo T, Nishida N, Itoh K. Transcatheter arterial embolization for tumor seeding in the chest wall after radiofrequency ablation for hepatocellular carcinoma. Cardiovasc Intervent Radiol. 2006;29:479–481. doi: 10.1007/s00270-004-0107-4. [DOI] [PubMed] [Google Scholar]
- 21.Wu CC, Chen WS, Ho MC, Huang KW, Chen CN, Yen JY, Lee PH. Minimizing abdominal wall damage during high-intensity focused ultrasound ablation by inducing artificial ascites. J Acoust Soc Am. 2008;124:674–679. doi: 10.1121/1.2839907. [DOI] [PubMed] [Google Scholar]
- 22.Robertson JD, de la Torre JI, Gardner PM, Grant JH, Fix RJ, Vásconez LO. Abdominoplasty repair for abdominal wall hernias. Ann Plast Surg. 2003;51:10–16. doi: 10.1097/01.SAP.0000054240.21252.64. [DOI] [PubMed] [Google Scholar]
- 23.Chang S, Kim SH, Lim HK, Kim SH, Lee WJ, Choi D, Kim YS, Rhim H. Needle tract implantation after percutaneous interventional procedures in hepatocellular carcinomas: lessons learned from a 10-year experience. Korean J Radiol. 2008;9:268–274. doi: 10.3348/kjr.2008.9.3.268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Liu FY, Yu XL, Liang P, Wang Y, Zhou P, Yu J. Comparison of percutaneous 915 MHz microwave ablation and 2450 MHz microwave ablation in large hepatocellular carcinoma. Int J Hyperthermia. 2010;26:448–455. doi: 10.3109/02656731003717574. [DOI] [PubMed] [Google Scholar]
- 25.Yu J, Liang P, Yu X, Wang Y, Gao Y. Ultrasound-guided percutaneous microwave ablation of splenic metastasis: report of four cases and literature review. Int J Hyperthermia. 2011;27:517–522. doi: 10.3109/02656736.2011.563768. [DOI] [PubMed] [Google Scholar]
- 26.Liang P, Gao Y, Zhang H, Yu X, Wang Y, Duan Y, Shi W. Microwave ablation in the spleen for treatment of secondary hypersplenism: a preliminary study. AJR Am J Roentgenol. 2011;196:692–696. doi: 10.2214/AJR.10.4193. [DOI] [PubMed] [Google Scholar]
- 27.Wang Y, Liang P, Yu X, Cheng Z, Yu J, Dong J. Ultrasound-guided percutaneous microwave ablation of adrenal metastasis: preliminary results. Int J Hyperthermia. 2009;25:455–461. doi: 10.1080/02656730903066608. [DOI] [PubMed] [Google Scholar]
- 28.Yu MA, Liang P, Yu XL, Cheng ZG, Han ZY, Liu FY, Yu J. Sonography-guided percutaneous microwave ablation of intrahepatic primary cholangiocarcinoma. Eur J Radiol. 2011;80:548–552. doi: 10.1016/j.ejrad.2011.01.014. [DOI] [PubMed] [Google Scholar]
- 29.Liang P, Wang Y, Zhang D, Yu X, Gao Y, Ni X. Ultrasound guided percutaneous microwave ablation for small renal cancer: initial experience. J Urol. 2008;180:844–848; discussion 848. doi: 10.1016/j.juro.2008.05.012. [DOI] [PubMed] [Google Scholar]
- 30.Dupuy DE. Image-guided thermal ablation of lung malignancies. Radiology. 2011;260:633–655. doi: 10.1148/radiol.11091126. [DOI] [PubMed] [Google Scholar]
- 31.Espinoza S, Briggs P, Duret JS, Lapeyre M, de Baère T. Radiofrequency ablation of needle tract seeding in hepatocellular carcinoma. J Vasc Interv Radiol. 2005;16:743–746. doi: 10.1097/01.RVI.0000153109.56827.70. [DOI] [PubMed] [Google Scholar]
- 32.Simon CJ, Dupuy DE, Mayo-Smith WW. Microwave ablation: principles and applications. Radiographics. 2005;25 Suppl 1:S69–S83. doi: 10.1148/rg.25si055501. [DOI] [PubMed] [Google Scholar]
- 33.Kuang M, Lu MD, Xie XY, Xu HX, Mo LQ, Liu GJ, Xu ZF, Zheng YL, Liang JY. Liver cancer: increased microwave delivery to ablation zone with cooled-shaft antenna--experimental and clinical studies. Radiology. 2007;242:914–924. doi: 10.1148/radiol.2423052028. [DOI] [PubMed] [Google Scholar]
- 34.Carrafiello G, Laganà D, Mangini M, Fontana F, Dionigi G, Boni L, Rovera F, Cuffari S, Fugazzola C. Microwave tumors ablation: principles, clinical applications and review of preliminary experiences. Int J Surg. 2008;6 Suppl 1:S65–S69. doi: 10.1016/j.ijsu.2008.12.028. [DOI] [PubMed] [Google Scholar]
- 35.Boutros C, Somasundar P, Garrean S, Saied A, Espat NJ. Microwave coagulation therapy for hepatic tumors: review of the literature and critical analysis. Surg Oncol. 2010;19:e22–e32. doi: 10.1016/j.suronc.2009.02.001. [DOI] [PubMed] [Google Scholar]