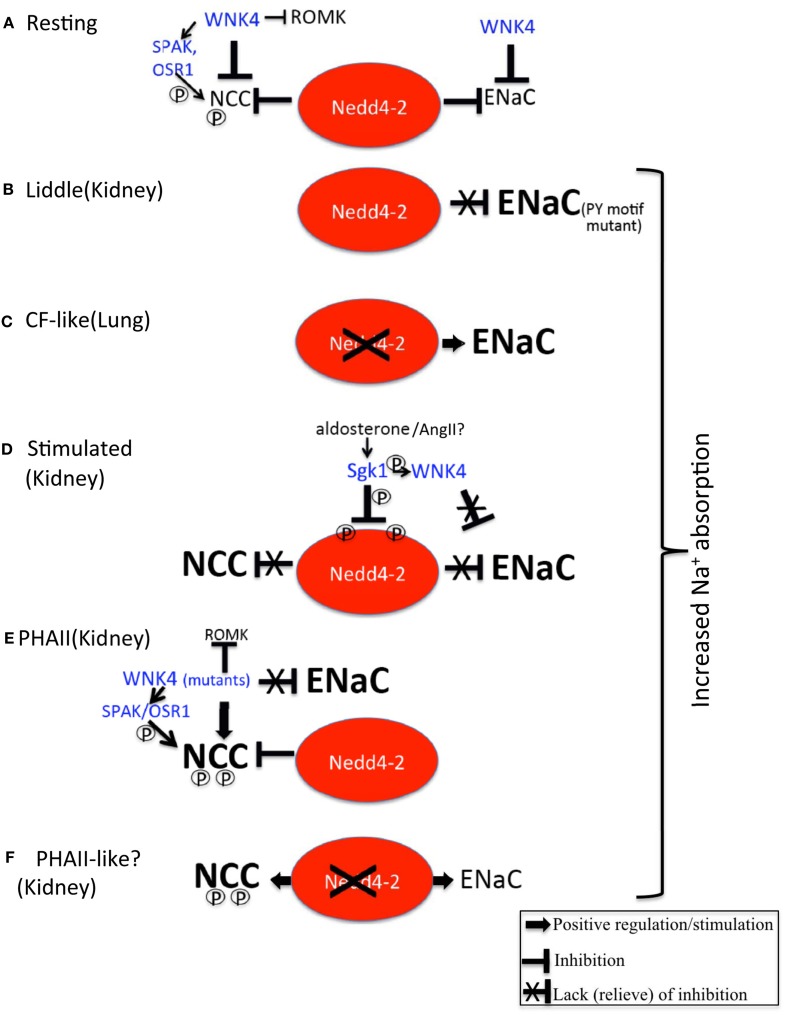
Figure 1.
Regulation of ENaC and NCC by Nedd4-2. (A) Under resting conditions, Nedd4-2 inhibits ENaC and NCC, as does WNK4, ensuring no excess Na+ (and fluid) reabsorption takes place, especially in the kidney. An alternative model suggests that WNK4 activates NCC to a low degree by activating the kinases SPAK and OSR1, which then phosphorylate (P) NCC. WNK4 also inhibits the K+ channel ROMK, to restrain K+ excretion. (B) In Liddle syndrome, PY motif mutations in ENaC prevent Nedd4-2 from properly binding and suppressing ENaC, thus leading to increased ENaC abundance/function and excessive Na+ (and fluid) reabsorption in the kidney, causing hypertension. (C) Removal of Nedd4-2 in lung epithelia by gene knockout in mice leads to cystic fibrosis – like lung disease due to excessive abundance/function of ENaC. (D) Aldosterone (or Angiotensin II, AngII?) stimulation of Sgk1 leads to Sgk1-mediated phosphorylation of Nedd4-2 on two sites, thus preventing Nedd4-2 from suppressing NCC and ENaC. This results in increased abundance/function of these Na+ absorbing proteins. In addition, phosphorylation of WNK4 by Sgk1 interferes with the ability of WNK4 to inhibit ENaC. Collectively, this leads to aldosterone-mediated increased in Na+ and fluid reabsorption in the kidney, hence to elevated blood pressure. (E) In PHAII, WNK4 mutants either divert NCC to the recycling pathway instead of lysosomal degradation, and/or enhance phosphorylation (and activation) of SPAK/OSR1 and hence of NCC. Both of these effects, coupled with loss of inhibition of ENaC by WNK4, lead to increased Na+ reabsorption (and paracellular Cl− permeability) in the kidney, and hence to increased fluid absorption and hypertension. Mutant WNK4 also strongly suppresses ROMK, preventing K+ excretion and causing hyperkalemia. (F) Knockout of Nedd4-2 in the distal nephron leads to increased abundance and activation of NCC (and ENaC), causing hypertension and possibly mimicking features of PHAII.