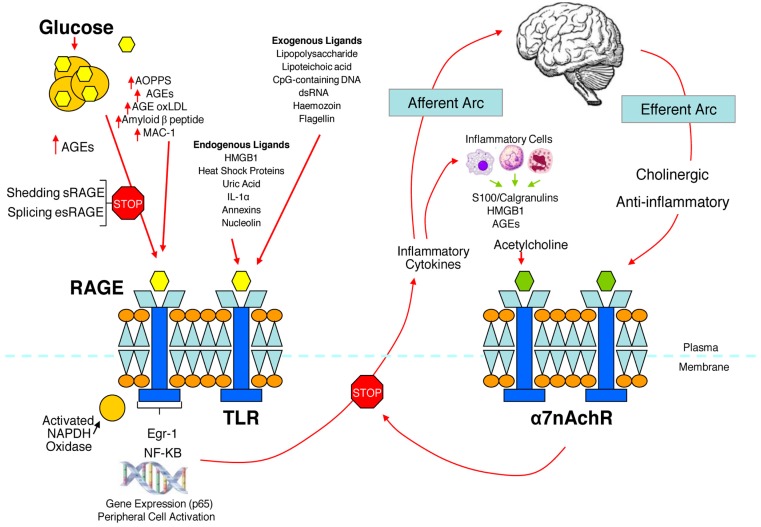
Figure 3.
The relationship between binding of ligands to the pattern recognition AGE receptor (RAGE) and inflammation, gene expression, oxidative and nitrosative stress, and damage to the macro- and microvasculature. Elevated levels of glucose bind to proteins and form AGEs, which bind to RAGEs. RAGE signaling activates NADPH oxidase and production of reactive oxygen species (ROS). Increased ROS increases advanced oxidation protein products (AOPPs), more AGEs, and AGE-modification of oxidized LDLs (oxLDLs). Furthermore, increased ROS may deplete glutathione, thereby suppressing glyoxalase I activity, a mechanism favoring further AGE accumulation. AGEs, AOPPs, macrophage glycoprotein (MAC-1), and AGE-oxLDL ligands of RAGE sustain stimulation of RAGE, and these processes, together with increased ROS, activate key transcription factors such as nuclear factor-κB (NF-κB) and Egr-1, which increase gene transcription factors and activate inflammatory mechanisms. Consequences include increased migration and activation of RAGE-expressing neutrophils, monocytes/macrophages, T-cells, and dendritic cells. This results in the release of the pro-inflammatory RAGE ligands S100/calgranulins and high-mobility group protein box-1 (HMGB1). In this inflammatory environment, further AGEs may be formed as well. Via interaction with RAGE, these ligands magnify activation of NF-κB, Agr-1, and other factors, thereby amplifying cellular stress and tissue damage leading to neurovascular dysfunction. Soluble RAGE (sRAGE) is formed from the cleavage of RAGE by disintegrins such as ADAM 10, a metalloproteinase, and β- and γ-secretases. sRAGE or a spliced variant (esRAGE) compete for binding of ligands to RAGE, and a deficiency could theoretically initiate the sequence of events activating an inflammatory cascade with an increase in the expression of pro-inflammatory cytokines [E-selectin, endothelin-1 tissue factor, vascular endothelial growth factor, and other pro-inflammatory cytokines (interleukin-6 and tumor necrosis factor-α)] and damage to neurons, kidney, eye, the vasculature, and even bone. Increasing sRAGE or its administration could competitively reduce activation of the AGE/RAGE pathway and it consequences. In addition endogenous and exogenous ligands bind to Toll-like receptors (TLR) also targeting NF-KB as well as inducing the secretion of pro-inflammatory cytokines which activate afferent sensory neurons reaching the brainstem via axons in the vagus. This in turn activates the cholinergic anti-inflammatory efferent arc which inhibits response in cytokine producing immune cells and signal through the nicotinic acetylcholine receptor subunit α7 (α7nACHR). This in turn inhibits NF-κB activation. Thus the pathways of AGE/RAGE activation of the inflammatory cascade and the inflammatory ligands target activation of an inflammatory cascade that can be abrogated by either competing with the binding of ligands to RAGE or by vagal activation of the anti-inflammatory reflex. Thus central to curtailing unbridled activation of the inflammatory cascade is the integrity of parasympathetic autonomic function or balance between the two arms of the autonomic nervous system.