Abstract
The default mode network (DMN) of the brain consists of areas that are typically more active during rest than during active task performance. Recently however, this network has been shown to be activated by certain types of tasks. Social cognition, particularly higher-order tasks such as attributing mental states to others, has been suggested to activate a network of areas at least partly overlapping with the DMN. Here, we explore this claim, drawing on evidence from meta-analyses of functional MRI data and recent studies investigating the structural and functional connectivity of the social brain. In addition, we discuss recent evidence for the existence of a DMN in non-human primates. We conclude by discussing some of the implications of these observations.
Keywords: default mode network, mentalizing, social cognition, fMRI, theory of mind, TPJ, posterior cingulate, medial frontal cortex
Default mode or social brain?
When human participants are not engaged in any specific task a set of brain regions can be observed to be active (Shulman et al., 1997). Collectively this set of regions is now commonly referred to as the “default mode network” (DMN; Raichle et al., 2001; Buckner et al., 2008). Regions of the DMN often show a deactivation when participants perform cognitive tasks and this degree of deactivation can be predictive of subsequent performance (Eichele et al., 2008). Contributing to its popularity, the DMN is easy to identify in resting state functional MRI data and its integrity seems to be compromised in a number of neurological and psychiatric syndromes, such as Alzheimer's disease (Hafkemeijer et al., 2012) and autism (Anderson et al., 2011).
It is now becoming increasingly apparent that brain areas associated with the DMN are activated during the performance of certain types of tasks. Indeed, the proposed anti-correlation between the DMN and task-related networks has been called into question (Murphy et al., 2009; Kelly et al., 2012). In this paper, we will focus on one of the task domains that has been suggested to activate the DMN, that of social cognition. It has been observed that there is strong overlap between the network of areas activated in social cognition and the DMN (Corbetta et al., 2008; Schilbach et al., 2008), but further systematic investigation has been lacking. We will explore the hypothesis that areas in the DMN are involved in certain types of social cognition, speculate on why this might be the case, and explore its implications for understanding social cognition not just in humans, but also in non-human primates.
Importantly, in this paper we attempt to take this discussion further by reviewing some recent anatomical data on the DMN and areas involved in social cognition, since a better understanding of the underlying anatomy is vital to understanding a brain network's function. Second, we discuss recent data on the anatomical relation between the DMN and social cognition networks in non-human primates providing independent evidence for a degree of overlap between DMN and brain areas mediating social cognition. We conclude by proposing some hypotheses on why certain aspects of social cognition might rely on the DMN and the consequences of this finding.
The default mode network
The history of research into the DMN is described in detail by a number of papers (Raichle and Snyder, 2007; Buckner et al., 2008), so we will only provide a brief overview here. The DMN was originally identified in block-design positron emission tomography (PET) studies by looking at brain areas that showed activity increases during passive or “rest” blocks compared to “task” blocks (Shulman et al., 1997). Following a series of influential papers by Raichle and colleagues, the DMN emerged as its own area of research (Gusnard et al., 2001; Raichle et al., 2001). These papers argued that the DMN identified by task-negative contrasts is a specific anatomical network, distinguishable from task-negative effects in unattended sensory modalities related to attention (Haxby et al., 1994).
More recently, the DMN is often identified in pure “resting state” experiments. In this type of paradigm, participants' brain activity is recorded while participants are not performing any task and are usually left undirected to think for themselves. By extracting the time course in a region of interest and correlating that with brain activity at each voxel, one can obtain a map of functional interactions between brain areas during rest. Seeding, for instance, the posterior cingulate cortex (PCC) results in a map of DMN (Greicius et al., 2002). Alternatively, the data can be analyzed using model-free analysis techniques such as independent component analysis (ICA), which allow one to characterize the spatio-temporal structure of the data (Beckmann et al., 2005). This results in a number of independent components, each reflecting a distinct network of interacting brain regions, in which the DMN is often captured in a single, or very few, components.
A number of areas are consistently found regardless of the method used, although some differences have been identified as well. Areas consistently identified are the medial posterior cortex, specifically posterior cingulate cortex (PCC; areas 23/31) and often the precuneus, the medial frontal cortex (MFC; including areas 24/10-m/32), and bilateral inferior parietal and posterior temporal areas around the temporoparietal junction area (TPJ). Apart from these core nodes, other areas that are often reported to participate in the DMN are the hippocampal formation and medial temporal lobe and areas along the lateral temporal cortex extending toward the temporal pole.
In the human brain diffusion-weighted imaging has been used to identify the cingulum bundle as an important white matter tract mediating the functional connectivity between two of the core hubs of the DMN, the posterior and anterior medial cortices (van den Heuvel et al., 2008; Greicius et al., 2009). Indeed, the areas of the DMN are generally heavily interconnected with one another. It has been argued that some of these regions form part of the “structural core” of the neocortex, consisting of nodes linking all main major structural modules of the brain (Hagmann et al., 2008). Finally, based on a detailed analysis of the resting state functional connectivity patterns it has been suggested that the DMN comprises at least two subsystems, one including the lateral temporal cortex, temporal pole, and dorsomedial frontal cortex, and another one centered on the medial temporal lobe, hippocampal formation, posterior inferior parietal lobule (IPL), and ventral MFC (Andrews-Hanna et al., 2010).
Overlap between the default mode network and social brain networks
One of the first studies to explore the relationship between the DMN and the neural basis of social cognition was performed by Schilbach and colleagues (Schilbach et al., 2008). They performed a conjunction analysis on the data from 12 studies from their lab, defining the DMN by looking for areas that correlated negatively with the task-related regressors defined in these studies. Their analysis revealed the left angular gyrus, the precuneus, and the ventral anterior cingulate cortex. The authors then noted that some of the activations were very similar to those observed in various aspects of social cognition from their lab and other groups, including the involvement of the precuneus in social interactions (Schilbach et al., 2006), the left angular gyrus/TPJ in differentiating between self and others (Vogeley and Fink, 2003), and anterior cingulate in action monitoring in self and others (Amodio and Frith, 2006). The authors proposed that the physiological “baseline” of the human brain is linked to the psychological “baseline,” the predisposition human beings have for social cognition as the default mode of thought.
As outlined above, the DMN can also be identified, together with other functional networks, in fMRI data collected at rest. Recently, Smith and colleagues compared resting state networks as defined by ICA with networks of brain areas showing consistent co-activation during task performance using the activation maps of experiments included in the online BrainMap database (www.brainmap.org). They showed close correspondence between the networks described by independent components in the resting state and networks of co-activating brain regions during experiments (Smith et al., 2009). In a subsequent study they went on to use the meta-data associated with each study in their meta-analysis to investigate the type of tasks that commonly activate each network. A network highly reminiscent of the DMN, showing bilateral inferior parietal/TPJ, precuneus/posterior cingulate, and medial frontal activation (their component 13, see also Figure 1A) loaded strongly and exclusively on only one behavioral domain, that of social cognition (Laird et al., 2011).
Figure 1.
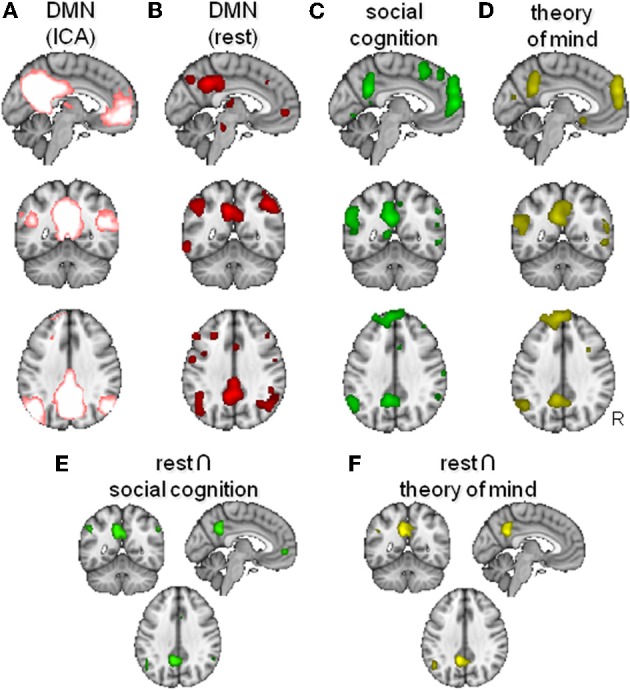
Overlap between the default mode network (DMN) and areas activated by social cognition paradigms. (A) DMN as found using model-free analysis of resting state fMRI data (Smith et al., 2009). (B,C) Activation likelihood maps of activity during passive “rest” conditions (B), social cognition (C) and theory of mind (D). (E,F) Conjunction maps of rest and social cognition (E) and of rest and theory of mind (F).
To further investigate the overlap between the DMN and the activity during social cognition tasks, we performed an additional meta-analysis of fMRI studies using the BrainMap database and performed likelihood estimations (Eickhoff et al., 2009) of functional brain activity associated with rest and associated with social cognition. The BrainMap database was queried on Jan 30th, 2012, when the database contained 2177 papers, 83 paradigm classes, 40934 participants, 10330 experiments, and 82135 locations. Focusing only on studies in healthy participants (Subjects: Diagnosis is Normals), we performed three different searches.
First, we defined the network of regions active during rest by asking for activations during experiments were participants we instructed to remain passive (Conditions: Instruction is Passive/Rest AND Subjects: Diagnosis is Normals), which yielded 485 papers, with 1648 of 2417 experiments matching criteria. This analysis yielded foci in the posterior medial, anterior medial, and lateral temporoparietal cortices (Figure 1B). This network of regions is very similar to the DMN defined by ICA of resting state data by Smith et al. (2009) as displayed in Figure 1A.
Second, we investigated activations related to the broad domain of social cognition (Experiments: Behavioral Domain is Cognition: Social Cognition AND Subjects: Diagnosis is Normals), which yielded 52 papers, with 186 of 216 experiments matching criteria. This analysis (Figure 1C) yielded results very similar to those described above for the resting state, including medial frontal, posterior cingulate, and lateral temporoparietal foci. Although the activation maps of the rest and the social cognition studies seem generally very similar, lateral temporoparieal activation seemed to be extending more dorsally during rest. In contrast, social cognition tended to activate a larger extend of medial frontal cortex. A conjunction between the DMN defined in the first analysis and these social cognition foci (thresholded >100 voxels) showed significant overlap in the anterior (para)cingulate bilateral, left and right angular gyrus, left frontal operculum, and the posterior cingulate extending into the precuneus (Figure 1E).
The domain of social cognition is of course rather broad, comprising processes such as obtaining, retrieving, and processing information about the lifes, relationships, and mental states of the self and others. A network of DMN areas, including TPJ and MFC has been attributed a role in mentalizing or Theory of Mind, i.e., the ability to understand and manipulates the beliefs of others (Hagmann et al., 2008). This faculty is argued to be particularly well developed in humans as compared to other primates (Saxe, 2006). Therefore, in a final analysis, we looked more specifically at activations related to this domain of social cognition by searching for activations related to theory of mind (Experiments: Paradigm Class is Theory of Mind Task AND Subjects: Diagnosis is Normals), which yielded 24 papers, with 99 of 124 experiments matching criteria. Again, a similar network was noticeable (Figure 1D), although to a lesser extent. A conjunction between the DMN defined in the first analysis and the theory of mind network (thresholded > 100 voxels) showed significant overlap in the left angular gyrus and the posterior cingulate, again extending into the precuneus (Figure 1F).
Nodes of the DMN in social cognition
Having established that there is some global overlap between the networks identified as the “DMN” and those active during certain social cognitive tasks, we will now focus on the different nodes of these networks in a bit more detail. We will concentrate on the three nodes of the DMN most consistently reported: medial frontal cortex, medial posterior cortex, and lateral temporoparietal areas.
Increased activity in the medial posterior cortex was one of the first and most robust findings in the default mode literature. A substantial number of studies refer to this locus as posterior cingulate and precuneus. However, a recent study argued that only the PCC, area 23/31, has a connectivity pattern reminiscent of the DMN and that the precuneus, area 7-m, should therefore not be considered part of the default mode (Margulies et al., 2009). This study showed functional interactions in the resting state of PCC with ventral and dorsal prefrontal regions, medial temporal cortex, and lateral inferior parietal and temporal cortex. In the domain of social cognition, PCC has been attributed a role in attributing mental states to others (Saxe and Powell, 2006). Medial frontal regions belonging to the DMN have been less consistently characterized, with foci having been reported ventrally in the medial area 10 and dorsally in areas 32 and 24. However, the precise organization of the human medial frontal cortex and its similarity with the macaque MFC remains a topic of debate (Beckmann et al., 2009). In contrast to the PCC, medial frontal regions have been argued to have more generalized roles in social cognition, beyond the specific attribution of mental states (Saxe, 2006). The ventral part of the medial frontal cortex is commonly seen in tasks probing empathy and gray matter in this area correlates with mentalizing abilities and social network size (Lewis et al., 2011).
Apart from these medial areas, the most commonly identified regions of the DMN are bilateral areas along the posterior IPL, often extending into posterior superior temporal cortex. This area includes a region that in the literature on the neural basis of social cognition is often referred to as the TPJ. Indeed, it has been argued that the TPJ is the area most associated with theory of mind tasks or mentalizing (Saxe, 2006). However, the precise locus of this “social TPJ” remains a topic of debate, complicating any comparison of functional anatomies across domains. Indeed, there is an ongoing debate on whether activations in the TPJ related to theory of mind and those related to other cognitive processes, such as attentional switching are in the same or different cortical areas (Decety and Lamm, 2007; Mitchell, 2008; Scholz et al., 2009).
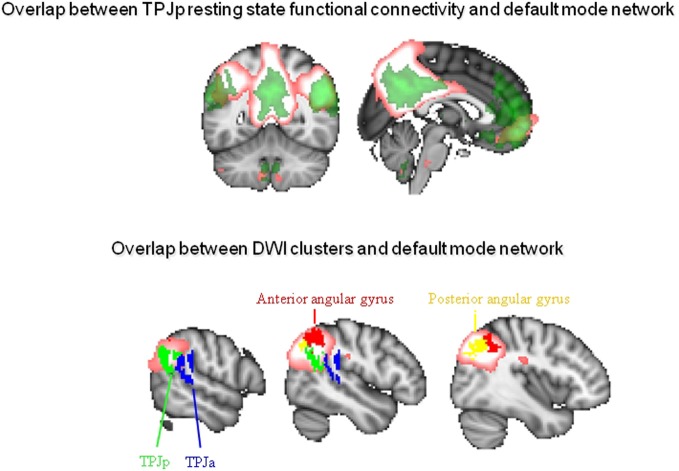
We have recently performed two studies aimed at characterizing the lateral parietal cortex and TPJ in the human brain. We used a combination of techniques. First, we used diffusion imaging, an MRI technique aimed at characterizing the white matter pathways connecting areas of the brain (Johansen-Berg and Rushworth, 2009), to parcellate these brain areas into subregions based on their structural connectivity with the whole brain. Second, we used resting state fMRI to investigate which larger cortical networks the resulting regions are part of. In the first study, we parcellated the human right lateral parietal cortex, focusing specifically on comparing its general organization with that of the macaque monkey (Mars et al., 2011). We showed a general similarity in organization between the human and macaque parietal cortex, with some differences in the strength of connections with the prefrontal cortex. In this study, we subdivided the IPL into five separate regions, organized into a posterior-to-anterior arrangement. This organization was highly similar to that suggested by previous cytoarchitectonic parcellations in the human brain (Caspers et al., 2008) and showed general similarities to that of the macaque IPL. In the second study, we focused on the right TPJ in the human brain, an area whose exact anatomical location and connectivity have been poorly characterized. We parcellated a large area of interest incorporating all the locations that have been described as “TPJ” in the literature and separated it into three components (Mars et al., in press). We reported a dorsal area and two ventral regions. The dorsal area overlapped with the IPL regions in our parietal study (Mars et al., 2011). Ventrally, we identified two areas, one anterior (TPJa) and one posterior (TPJp). We believe the posterior TPJ region overlaps with the foci traditionally associated with social cognition.
In order to investigate which larger cortical network each of the TPJ clusters participates in, we next investigated the resting state functional connectivity of each of them. We observed that the TPJp showed coupling with regions along the medial surface, including posterior cingulate/precuneus and areas in the vicinity of the anterior cingulate cortex/paracingulate gyrus. These areas are strongly reminiscent of the DMN. To explore this issue, we used ICA to identify the default mode in the same dataset and plotted some of the regions identified in the parietal and TPJ studies on the same brain. As can be seen in Figure 2, the border between the anterior and posterior ventral TPJ subdivisons coincided with the extent of the DMN independent component. Moreover, the DMN component overlapped with the most posterior IPL subdivisions, the posterior and anterior parts of the angular gyrus. In this respect, it is interesting to point out that some posterior IPL regions might be similar to macaque area Opt, which has strong connections to the PCC and the limbic system (Caspers et al., 2011).
Figure 2.
Overlap between connectivity-based subregions of the inferior parietal lobule and temporoparietal junction area and the DMN. Top: Overlap between resting state function connectivity of the posterior TPJ as defined by Mars et al. (in press) in green and the DMN as defined using independent component analysis in pink. Bottom: Overlap between the DMN in pink and the anterior (TPJa) and posterior (TPJp) areas from Mars et al. (in press) and the anterior and posterior angular gyrus from Mars et al. (2011).
In summary, both at the level of the network and that of individual brain areas there seems to be a consistent overlap between the DMN and areas that are active during certain types of social cognitive tasks, most notably mentalizing tasks. Although the precise anatomy of both the DMN and the social brain are only partially mapped out, the core nodes of the DMN have all received tentative labels in terms of their contribution to social cognition. The challenge for the future is to further identify and localize these nodes and to determine tractable computations that each area is performing.
DMN and the social brain in non-human primates
An exciting development which might prove beneficial to understanding the relationship between social cognition and the DMN is that the DMN has now been reported in a number of non-human primates. Vincent and colleagues showed that macaque monkeys (Macaca mulatta and Macaca fascicularis) exhibit spontaneous brain activity similar to the human resting state while being scanned under light anesthesia (Vincent et al., 2007). When they seeded an area in the medial posterior cortex, probably enclosing the posterior cingulate and parts of the precuneus, a network consisting of posterior lateral and medial frontal activity emerged, similar to the human DMN. Following this result, Kojima and colleagues used PET in awake monkeys to show task-related deactivations in macaque medial cortical areas (Kojima et al., 2009). Although some differences with the human DMN were apparent their results provided further indication that monkeys have a DMN similar to that in the human brain. Building on this work, Mantini and colleagues recently performed a meta-analysis of 15 fMRI studies on awake macaques (Mantini et al., 2011). Similar to the approach taken in Schilbach's 2008 paper on humans, they looked at areas of the brain that were active during the rest periods as opposed to task blocks in these studies. The authors tentatively suggested that the macaque DMN included areas in the medial and lateral prefrontal cortex, posterior cingulate areas, and lateral inferior parietal and temporal-occipital regions. The authors also noted the possible existence of different subsystems within the macaque DMN, one consisting of the temporo-parietal-occipital cortex, MFC, and area 8b, and one consisting of posterior cingulate and inferior parietal areas. Beyond these results in macaques, suggestions of brain activity associated with rest reminiscent of the human DMN has also been reported in chimpanzees (Rilling et al., 2007).
In light of these results showing that networks reminiscent of the DMN can be found in primates other than humans, it is interesting to establish if there is any relationship between the DMN and areas involved in social cognition in these species. Research into the neural basis of social cognition in non-human primates has generated considerable interest recently, amongst others due to the prominence of the so-called “social brain hypothesis” (Dunbar, 1998). This hypothesis relates the relative size of the primate brain to challenges associated with living in complex social groups. In order to test the hypothesis that it is the DMN that mediates some of these animals' social abilities, in would be necessary to relate differences in DMN organization with differences in social abilities between these species. Unfortunately, studies comparing the neural basis of social abilities between species remain rare (Rilling et al., 2012). Instead, some recent studies have focused on the effects of sociality on brain size between individuals of the same species.
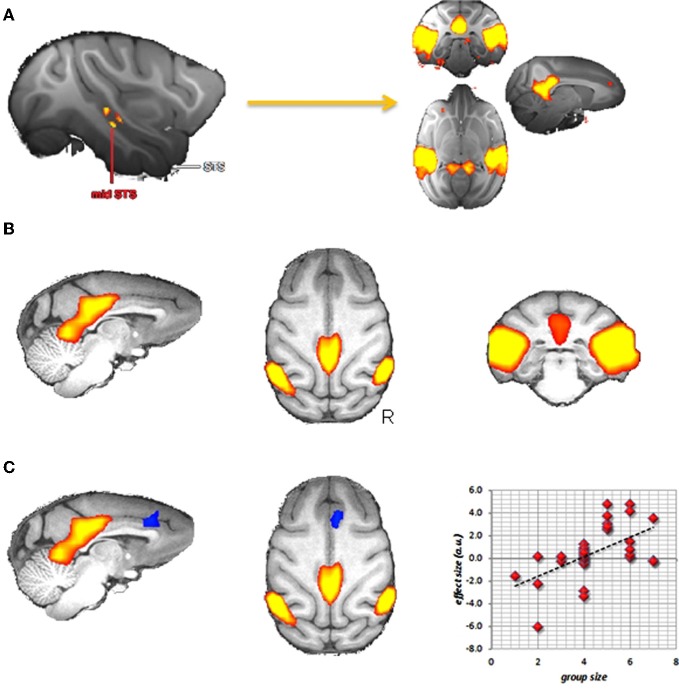
Sallet et al. (2011) investigated whether there are areas in the macaque (Macaca mulatta) brain that show structural differences in relationship to two factors describing the social life of captive macaques: the number of animals they are housed with and an animal's position in the group's social hierarchy. They reported a network of regions, including the rostral prefrontal cortex, amygdala, and anterior and middle superior temporal sulcus (STS), which showed increased gray matter when macaques were housed with more animals. Some of these regions, including rostral prefrontal cortex and inferior temporal cortex also showed increased gray matter in animals that occupied a higher position in the social hierarchy. The authors also acquired resting state fMRI in those animals. Using these data, we can look at areas of the brain showing functional connectivity with the areas showing gray matter differences related to social network size. Using the mid-STS region as a seed this results in posterior cingulate, anterior cingulate cortex, and lateral posterior areas (Figure 3A). This network is very similar to Vincent's DMN in macaques, indicating that the overlap between the DMN and brain areas involved in social cognition potentially extends beyond the human brain.
Figure 3.
Social brain in the macaque? (A) Areas in the macaque showing resting state functional connectivity with a region in the middle superior temporal sulcus that, in turn, showed increases in gray matter density in individuals living in larger social groups (data from Sallet et al., 2011). (B) Group independent component capturing the default mode network. (C) Dual regression results showing a region of the medial frontal cortex (in blue) that in increasingly recruited into the DMN (in red) when animals live in bigger groups.
We can then take this analysis a step further and see if any of these functional correlations with mid-STS are modulated by social network size. Sallet and colleagues did precisely this, and reported increased functional coupling between mid-STS and the anterior cingulate with increasing network size (Sallet et al., 2011), suggesting that the anterior cingulate is preferentially recruited into the DMN in participants with larger social networks. Going even further, we can test this hypothesis of increased involvement of the anterior cingulate cortex in the DMN directly. Using the recently established technique of dual-regression (Filippini et al., 2009) it is possible to test for individual differences in recruitment of brain areas in any particular resting state network. We used this technique on resting state data obtained from 32 macaque monkeys including those in the study of Sallet et al. (2011). From a group-ICA analysis, we selected the component that best captured the DMN (Figure 3B) and asked if there were any voxels in the brain that participate more in this component in participants housed with an increasing number of other animals. As can be seen in Figure 3C, the medial frontal cortex is increasingly recruited in the DMN in these monkeys. These results thus provide direct evidence that the DMN differs in individuals as a function of social network size.
It is informative to discuss some of the similarities and differences in the results obtained from humans and non-human primates. At first glance, the results in human and monkeys are very convergent. Both have a DMN consisting of medial frontal and parietal cores and lateral temporoparietal areas. Moreover, effects of sociality, operationalized by social network size and mentalizing ability have been shown to correlate with gray matter density in areas of the human brain (Bickart et al., 2011; Lewis et al., 2011; Dunbar, 2012), similar to the results obtained in macaques (Sallet et al., 2011). However, there are also differences, both in the anatomy of the two brains and, of course, in the social abilities of the two species (Passingham, 2008; Cheney, 2011). Some of the most notable anatomical differences are in regions reported in the gray matter density studies of social ability. For instance, in their analysis of the macaque DMN Mantini et al. (2011) noted that there is still uncertainty about the relationship between the MFC in humans and macaques. Furthermore, activity around the TPJ is commonly reported in the human DMN, but seed-based correlation analysis of the macaque DMN often show slightly more ventral areas in posterior lateral STS, which would be consistent with known connectivity of the macaque PCC (Kobayashi and Amaral, 2003). Given the large relative expansion of the middle parts of both the IPL and the STS in the human as compared to the macaque brain (van Essen and Dierker, 2007) some changes in relative position of these lateral nodes of the DMN might be expected. This, however, remains to be investigated in detail.
Discussion
In summary, we have investigated evidence for overlap between the DMN and areas involved in social cognition. We have shown that both at the network level and at the level of individual brain regions there is overlap between these two networks. We have highlighted the fact that the precise anatomical loci of areas involved in the DMN is not always known, particularly in the case of the area around the TPJ. Finally, we have investigated whether a similar relationship between brain areas involved in social skills and the DMN might be apparent in non-human primates. In what follows we will try to integrate these results and discuss their implications.
It is not the intention of this paper to claim that the purpose of the DMN is to “do” social cognition. Rather, the goal is to highlight the proposed overlap between the “social brain network” and the DMN and to discuss the potential implications of this, both with reference to the human and the wider primate literature. Although a number of authors have tried to recast social cognition in terms of underlying more basic processes, this has proven notoriously difficult (Behrens et al., 2008). The activity shown in areas commonly attributed to the DMN during social cognition provides an interesting challenge to find a common computational function for these two seemly very different functions and some studies are currently proposing frameworks for addressing this issue (Sadaghiani et al., 2010; Yoshida et al., 2010).
Studies trying to find order in the variety of processes that might be present when participants are “at rest” appeared from the beginning of the research into the DMN. Early studies already noted that “rest” consisted of a variety of functions (Andreasen et al., 1995), suggesting that rest might best be characterized as “Random Episodic Silent Thinking” about one's life and experiences. Since then, more and more reports have emerged of processes that seem to engage the DMN and there is a growing number of proposals regarding what the common denominator of these processes, is ranging from mind wandering (Mason et al., 2007) to the sense of self (Qin and Northoff, 2011).
Buckner et al. (2008) categorized these different hypotheses into two classes. The first class refers to hypotheses that emphasize the fact that the DMN is active during situations in which there is no strong task constraint, when participants are allowed or even encouraged to broadly monitor the environment, in contrast to the narrow tunnel vision often associated with psychological laboratory tasks. Consistent with this type of proposal, Platt and colleagues have recently suggested a more computationally constrained role of the posterior cingulate in the detection of changes in the environment and subsequent changes in decision policy and behavior (Pearson et al., 2011). The second class of hypotheses focuses on the involvement of the hippocampal and medial temporal structures in the DMN and attributes it a role more in mentation. In other words, processes which rely on episodic memory and mental simulations are prone to rely on the DMN. These hypotheses are broadly consistent with activity observed in the DMN during such processes as thinking about one's future and constructing a mental representation based on autobiographical memory (Spreng et al., 2009; Andrews-Hanna et al., 2010) and have also been cited as an important reason for the involvement of the DMN in social processes such as mentalizing. Both of these theories would be consistent with a function of the DMN in social cognition. It has been suggested that social cognition relies on processes that might be distinct from other forms of intelligence. Indeed, this “social function of intellect” hypothesis has been proposed repeatedly (Jolly, 1966; Humphrey, 1976) and forms the basis of the “social brain hypothesis” which states that our brains have expanded so much over the course of evolution precisely because of the challenges involved in living in large social groups (Dunbar, 1998). The ecology of social cognition might provide some clues as to why the DMN might have properties that are beneficial to this type of mental faculty. The largely unconstrained nature of social decision making, including its reliance on potentially multiple instances of recursive thinking might be one reason why social cognition relies on a network such as the DMN. As noted above, the DMN is characterized by the presence of a number of very rich nodes, i.e., areas that form long-range connections to other brain regions (Hagmann et al., 2008). Furthermore, it has been argued that the core skill necessary to survive in our complex social environments is the ability to keep track of the complex and constantly changing social relationships, not only of oneself with the other group members, but also between the other group members among themselves (De Waal, 1982; Cheney and Seyfarth, 2008). The presence of wide-range connections together with the subsystem involving areas associated with autobiographical memory might make the DMN a logical system to employ in social problem solving.
If it is true that higher-order social cognition relies at least in part on the DMN in the human brain, the question is what the finding of a similar network in chimpanzees and macaques means. At the very least, a strong reliance of higher order social cognition on an already existent neural basis would be consistent with theories which propose that great apes, and by extension the common precursor of great apes and humans, may have (had) many of the relevant cognitive preconditions for uniquely human social cognition to evolve. The “cooperative breeding hypothesis” proposed by Hrdy and colleagues (Burkart et al., 2009; Hrdy, 2009) suggests that it was the case, but that other apes and our common ancestor lacked the motivational preconditions that were required to developed full “human-style” mentalizing. In their specific hypothesis, it was the evolution of cooperative breeding, together with the existing ape-type brain, that lead to our complex social abilities (Burkart et al., 2009).
In conclusion, the overlap between the DMN and brain areas involved in social cognition deserves further attention. Its precise anatomy and computational function still harbors many unknowns, but their solution might have implications far beyond the field of cognitive neuroscience.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
The authors' research is supported by the Medical Research Council UK (G0802146, to Matthew F. S. Rushworth and Rogier B. Mars), the British Academy (SG110236 to Rogier B. Mars), the Nederlandse Organisatie voor Wetenschappelijk Onderzoek (NWO, 453.08.002 to Ivan Toni and 040.11.256 to Ivan Toni and Rogier B. Mars), and a Christopher Welch Scholarship (Franz-Xaver Neubert).
References
- Amodio D. M., Frith C. D. (2006). Meeting of minds: the medial frontal cortex and social cognition. Nat. Rev. Neurosci. 7, 268–277 10.1038/nrn1884 [DOI] [PubMed] [Google Scholar]
- Anderson J. S., Nielsen J. A., Froehlich A. L., DuBray M. B., Druzgal T. J., Cariello A. N., Cooperrider J. R., Zielinski B. A., Ravichandran C., Fletcher P. T., Alexander A. L., Bigler E. D., Lange N., Lainhart J. E. (2011). Functional connectivity magnetic resonance imaging classification of autism. Brain 134(Pt 12), 3742–3754 10.1093/brain/awr263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andreasen N. C., O'Leary D. S., Cizadlo T., Arndt S., Rezai K., Watkins G. L., Ponto L. L., Hichwa R. D. (1995). Remembering the past: two facets of episodic memory explored with positron emission tomography. Am. J. Psychiatry 152, 1576–1585 [DOI] [PubMed] [Google Scholar]
- Andrews-Hanna J. R., Reidler J. S., Sepulcre J., Poulin R., Buckner R. L. (2010). Functional-anatomic fractionation of the brain's default network. Neuron 65, 550–562 10.1016/j.neuron.2010.02.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beckmann C. F., DeLuca M., Devlin J. T., Smith S. M. (2005). Investigations into resting-state connectivity using independent component analysis. Philos. Trans. R. Soc. B Biol. Sci. 360, 1001–1013 10.1098/rstb.2005.1634 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beckmann M., Johansen-Berg H., Rushworth M. F. (2009). Connectivity-based parcellation of human cingulate cortex and its relation to functional specialization. J. Neurosci. 29, 1175–1190 10.1523/JNEUROSCI.3328-08.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behrens T. E., Hunt L. T., Woolrich M. W., Rushworth M. F. (2008). Associative learning of social value. Nature 456, 245–249 10.1038/nature07538 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bickart D., Wright C. I., Dautoff R. J., Dickerson B. C., Barrett L. F. (2011). Amygdala volume and social network size in humans. Nat. Neurosci. 14, 163–164 10.1038/nn.2724 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckner R. L., Andrews-Hanna J. R., Schacter D. L. (2008). The brain's default network. Anatomy, function, and relevance to disease. Ann. N.Y. Acad. Sci. 1124, 1–38 10.1196/annals.1440.011 [DOI] [PubMed] [Google Scholar]
- Burkart J. M., Hrdy S. B., Van Schaik C. P. (2009). Cooperative breeding and human cognitive evolution. Evol. Anthropol. 18, 175–186 [Google Scholar]
- Caspers S., Eickhoff S. B., Geyer S., Scheperjans F., Mohlberg H., Zilles K., Amunts K. (2008). The human inferior parietal lobule in stereotaxic space. Brain Struct. Funct. 212, 481–495 10.1007/s00429-008-0195-z [DOI] [PubMed] [Google Scholar]
- Caspers S., Eickhoff S. B., Rick T., Von Kapri A., Kuhlen T., Huang R., Shah N. J., Zilles K. (2011). Probabilistic fibre tract analysis of cytoarchitectonically defined human inferior parietal lobule areas revelas similarities to macaques. Neuroimage 58, 362–380 10.1016/j.neuroimage.2011.06.027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheney D. L. (2011). Extent and limits of cooperation in animals. Proc. Natl. Acad. Sci. U.S.A. 108, 10902–10909 10.1073/pnas.1100291108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheney D. L., Seyfarth R. M. (2008). Baboon Metaphysics: The Evolution of a Social Mind. Chicago, IL: University of Chicago Press [Google Scholar]
- Corbetta M., Patel G., Shulman G. L. (2008). The reorienting system of the human brain: from environment to theory of mind. Neuron 58, 306–324 10.1016/j.neuron.2008.04.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Waal F. (1982). Chimpanzee Politics: Power and Sex Among Apes. Baltimore, MD: Johns Hopkins University Press [Google Scholar]
- Decety J., Lamm C. (2007). The role of the right temporoparietal junction in social interaction: how low-level computational processes contribute to meta-cognition. Neuroscientist 13, 580–593 10.1177/1073858407304654 [DOI] [PubMed] [Google Scholar]
- Dunbar R. I. M. (1998). The social brain hypothesis. Evol. Anthropol. 6, 178–190 [Google Scholar]
- Dunbar R. I. M. (2012). The social brain meets neuroimaging. Trends Cogn. Sci. 16, 101–102 10.1016/j.tics.2011.11.013 [DOI] [PubMed] [Google Scholar]
- Eichele T., Debener S., Calhoun V. D., Specht K., Engel A. K., Hugdahl K., von Cramon D. Y., Ullsperger M. (2008). Prediction of human errors by maladaptive changes in event-related brain networks. Proc. Natl. Acad. Sci. U.S.A. 105, 6173–6178 10.1073/pnas.0708965105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eickhoff S. B., Laird A. R., Grefkes C., Wang L. E., Zilles K., Fox P. T. (2009). Coordinate-based activation likelihood estimation meta-analysis of neuroimaging data: a random-effects approach based on empirical estimates of spatial uncertainty. Hum. Brain Mapp. 30, 2907–2926 10.1002/hbm.20718 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filippini N., MacIntosh B. J., Hough M. G., Goodwin G. M., Frisoni G. B., Smith S. M., Matthews P. M., Beckmann C. F., Mackay C. E. (2009). Distinct patterns of brain activity in young carriers of the APOE-ε 4 allele. Proc. Natl. Acad. Sci. U.S.A. 106, 7209–7214 10.1073/pnas.0811879106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greicius M. D., Krasnow B., Reiss A. L., Menon V. (2002). Functional connectivity in the resting brain: a network analysis of the default mode hypothesis. Proc. Natl. Acad. Sci. U.S.A. 100, 253–258 10.1073/pnas.0135058100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greicius M. D., Supekar K., Menon V., Dougherty R. F. (2009). Resting-state functional connectivity reflects structural connectivity in the default mode network. Cereb. Cortex 19, 72–78 10.1093/cercor/bhn059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gusnard D. A., Akbudak E., Shulman G. L., Raichle M. E. (2001). Medial prefrontal cortex and self-referential mental activity: relation to a default mode of brain function. Proc. Natl. Acad. Sci. U.S.A. 98, 4259–4264 10.1073/pnas.071043098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hafkemeijer A., Van der Grond J., Rombouts S. A. (2012). Imaging the default mode network in aging and dementia. Biochem. Biophys. Acta 1822, 431–441 10.1016/j.bbadis.2011.07.008 [DOI] [PubMed] [Google Scholar]
- Hagmann P., Cammoun L., Gigandet X., Meuli R., Honey C. J., Wedeen V. J., Sporns O. (2008). Mapping the structural core of the human cerebral cortex. PLoS Biol. 6:e159 10.1371/journal.pbio.0060159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haxby J. V., Horwitz B., Ungerleider L. G., Maisog J. M., Pietrini P., Grady C. L. (1994). The functional organization of human extrastriate cortex: a PET-rCBF study of selective attention to faces and locations. J. Neurosci. 14, 6336–6353 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hrdy S. B. (2009). Mother and Others: The Evolutionary Origins of Mutual Understanding. Cambridge, MA: Bellknap Press [Google Scholar]
- Humphrey N. K. (1976). “The social function of intellect,” in Growing Pains in Ethology eds Bateson P. P. G., Hinde R. A. (Cambridge: Cambridge University Press; ), 303–317 [Google Scholar]
- Johansen-Berg H., Rushworth M. F. (2009). Using diffusion imaging to study human connectional anatomy. Annu. Rev. Neurosci. 32, 75–94 10.1146/annurev.neuro.051508.135735 [DOI] [PubMed] [Google Scholar]
- Jolly A. (1966). Lemur social behavior and primate intelligence. Science 153, 501–506 [DOI] [PubMed] [Google Scholar]
- Kelly C., Biswal B. B., Craddock R. C., Castellanos F. X., Milham M. P. (2012). Characterizing variation in the functional connectome: promise and pitfalls. Trends Cogn. Sci. 16, 181–188 10.1016/j.tics.2012.02.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi Y., Amaral D. G. (2003). Macaque monkey retrosplenial cortex: II. Cortical afferents. J. Comp. Neurol. 466, 48–79 10.1002/cne.10883 [DOI] [PubMed] [Google Scholar]
- Kojima T., Onoe H., Hikosaka K., Tsutsui K., Tsukada H., Watanabe M. (2009). Default mode of brain activity demonstrated by positron emission tomography imaging in awake monkeys: higher rest-related than working memory-related activity in medial cortical areas. J. Neurosci. 29, 14463–14471 10.1523/JNEUROSCI.1786-09.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laird A. R., Fox P. M., Eickhoff S. B., Turner J. A., Ray K. L., McKay D. R., Glahn D. C., Beckmann C. F., Smith S. M., Fox P. T. (2011). Behavioral interpretations of intrinsic connectivity networks. J. Cogn. Neurosci. 23, 4022–4037 10.1162/jocn_a_00077 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis P. A., Rezaie R., Brown R., Roberts N., Dunbar R. I. M. (2011). Ventromedial prefrontal volume predicts understanding of others and social network size. Neuroimage 57, 1624–1629 10.1016/j.neuroimage.2011.05.030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mantini D., Gerits A., Nelissen K., Durand J. B., Joly O., Simone L., Sawamura H., Wardak C., Orban G. A., Buckner R. L., VanDuffel W. (2011). Default mode of brain function in monkeys. J. Neurosci. 31, 12954–12962 10.1523/JNEUROSCI.2318-11.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Margulies D. S., Vincent J. L., Kelly C., Lohmann G., Uddin L. Q., Biswal B. B., Villringer A., Castellanos F. X., Milham M. P., Petrides M. (2009). Precuneus shares intrinsic functional architecture in humans and monkeys. Proc. Natl. Acad. Sci. U.S.A. 106, 20069–20074 10.1073/pnas.0905314106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mars R. B., Jbabdi S., Sallet J., O'Reilly J. X., Croxson P. L., Olivier E., Noonan M. P., Bergmann C., Mitchell A. S., Baxter M. G., Behrens T. E. J., Johansen-Berg H., Tomassini V., Miller K. L., Rushworth M. F. S. (2011). Diffusion-weighted imaging tractography-based parcellation of the human parietal cortex and comparison with human and macaque resting state functional connectivity. J. Neurosci. 31, 4087–4100 10.1523/JNEUROSCI.5102-10.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mars R. B., Sallet J., Schüffelgen U., Jbabdi S., Toni I., Rushworth M. F. S. (in press). Connectivity-based subdivisions of the human right ‘temporoparietal junction area’ (TPJ): evidence for different areas participating in different cortical networks. Cereb. Cortex. [Epub ahead of print]. 10.1093/cercor/bhr268 [DOI] [PubMed] [Google Scholar]
- Mason M. F., Norton M. I., Van Horn J. D., Wegner D. M., Grafton S. T., Macrae C. N. (2007). Wandering minds: the default network and stimulus-independent thought. Science 315, 393–395 10.1126/science.1131295 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell J. P. (2008). Activity in right temporo-parietal junction is not selective for theory-of-mind. Cereb. Cortex 18, 262–271 10.1093/cercor/bhm051 [DOI] [PubMed] [Google Scholar]
- Murphy K., Birn R. M., Handwerker D. A., Jones T. B., Bandettini P. A. (2009). The impact of global signal regression on resting state correlations: are anti-correlated networks introduced? Neuroimage 44, 893–905 10.1016/j.neuroimage.2008.09.036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Passingham R. (2008). What is Special About the Human Brain? Oxford, UK: Oxford University Press [Google Scholar]
- Pearson J. M., Hayden B. Y., Platt M. L. (2011). “A role for posterior cingulate in policy switching and cognitive control,” Neural Basis of Motivational and Cognitive Control, eds Mars R. B., Sallet J., Rushworth M. F. S., Yeung N. (Cambridge, MA: MIT Press; ), 127–143 [Google Scholar]
- Qin P., Northoff G. (2011). How is our self-related to midline regions and the default-mode network? Neuroimage 57, 1221–1233 10.1016/j.neuroimage.2011.05.028 [DOI] [PubMed] [Google Scholar]
- Raichle M. E., MacLeod A. M., Snyder A. Z., Powers W. J., Gusnard D. A., Shulman G. L. (2001). A default mode of brain function. Proc. Natl. Acad. Sci. U.S.A. 98, 676–682 10.1073/pnas.98.2.676 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raichle M. E., Snyder A. Z. (2007). A default mode of brain function: a brief history of an evolving idea. Neuroimage 37, 1083–1090 10.1016/j.neuroimage.2007.02.041 [DOI] [PubMed] [Google Scholar]
- Rilling J. K., Barks S. K., Parr L. A., Preuss T. M., Faber T. L., Pagnoni G., Bremner J. D., Votaw J. R. (2007). A comparison of resting-state brain activity in humans and chimpanzees. Proc. Natl. Acad. Sci. U.S.A. 104, 17146–17151 10.1073/pnas.0705132104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rilling J. K., Scholz J., Preuss T. M., Glasser M. F., Errangi B. K., Behrens T. E. (2012). Differences between chimpanzees and bonobos in neural systems supporting social cognition. Soc. Cogn. Affect. Neurosci. 7, 369–379 10.1093/scan/nsr017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sadaghiani S., Hesselmann G., Friston K. J., Kleinschmidt A. (2010). The relation of ongoing brain activity, evoked neural responses, and cognition. Front. Syst. Neurosci. 4:20 10.3389/fnsys.2010.00020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sallet J., Mars R. B., Noonan M. P., Andersson J., O'Reilly J. X., Jbabdi S., Croxson P. L., Miller K. L., Jenkinson M., Rushworth M. F. S. (2011). Social network size affects neural circuits in macaques. Science 334, 697–700 10.1126/science.1210027 [DOI] [PubMed] [Google Scholar]
- Saxe R. (2006). Uniquely human social cognition. Curr. Opin. Neurobiol. 16, 235–239 10.1016/j.conb.2006.03.001 [DOI] [PubMed] [Google Scholar]
- Saxe R., Powell J. T. (2006). It's the thought that counts: specific brain regions for one component of theory of mind. Psychol. Sci. 17, 692–699 10.1111/j.1467-9280.2006.01768.x [DOI] [PubMed] [Google Scholar]
- Schilbach L., Eickhoff S. B., Rotarska-Jagiela A., Fink G. R., Vogeley K. (2008). Minds at rest? Social cognition as the default mode of cognizing and its putative relationship to the default system of the brain. Conscious. Cogn. 17, 457–467 10.1016/j.concog.2008.03.013 [DOI] [PubMed] [Google Scholar]
- Schilbach L., Wohlschlaeger A. M., Kraemer N. C., Newen A., Shah N. J., Fink G. R., Vogeley K. (2006). Being with virtual others: neural correlates of social interaction. Neuropsychologia 44, 718–730 10.1016/j.neuropsychologia.2005.07.017 [DOI] [PubMed] [Google Scholar]
- Scholz J., Triantafyllou C., Whitfield-Gabrieli S., Brown E. N., Saxe R. (2009). Distinct regions of right temporo-parietal junction are selective for theory of mind and exogenous attention. PLoS ONE 4:e4869 10.1371/journal.pone.0004869 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shulman G. L., Fiez J. A., Corbetta M., Buckner R. L., Miezin F. M., Raichle M. E., Petersen S. E. (1997). Common blood flow changes across visual tasks. 2. Decreases in cerebral cortex. J. Cogn. Neurosci. 9, 648–663 [DOI] [PubMed] [Google Scholar]
- Smith S. M., Fox P. T., Miller K. L., Glahn D. C., Fox P. M., Mackay C. E., Filippini N., Watkins K. E., Toro R., Laird A. R., Beckmann C. F. (2009). Correspondence of the brain's functional architecture during activation and rest. Proc. Natl. Acad. Sci. U.S.A. 106, 13040–13045 10.1073/pnas.0905267106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spreng R. N., Mar R. A., Kim A. S. N. (2009). The common neural basis of autobiographical memory, prospection, navigation, theory of mind, and the default mode: a quantitative meta-analysis. J. Cogn. Neurosci. 21, 489–510 10.1162/jocn.2008.21029 [DOI] [PubMed] [Google Scholar]
- van den Heuvel M., Mandl R., Luigjes J., Hulshoff Pol H. (2008). Microstructural organization of the cingulum tract and the level of default mode functional connectivity. J. Neurosci. 28, 10844–10851 10.1523/JNEUROSCI.2964-08.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Essen D. C., Dierker D. L. (2007). Surface-based and probabilistic atlases of primate cerebral cortex. Neuron 56, 209–225 10.1016/j.neuron.2007.10.015 [DOI] [PubMed] [Google Scholar]
- Vincent J. L., Patel G. H., Fox M. D., Snyder A. Z., Baker J. T., Van Essen D. C., Zempel J. M., Snyder L. H., Corbetta M., Raichle M. E. (2007). Intrinsic functional architecture in the anaesthetized monkey brain. Nature 447, 83–86 10.1038/nature05758 [DOI] [PubMed] [Google Scholar]
- Vogeley K., Fink G. R. (2003). Neural correlates of the first-person-perspective. Trends Cogn. Sci. 7, 38–42 10.1016/S1364-6613(02)00003-7 [DOI] [PubMed] [Google Scholar]
- Yoshida W., Seymour B., Friston K. J., Dolan R. J. (2010). Neural mechanisms of belief inference during cooperative games. J. Neurosci. 30, 10744–10751 10.1523/JNEUROSCI.5895-09.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]