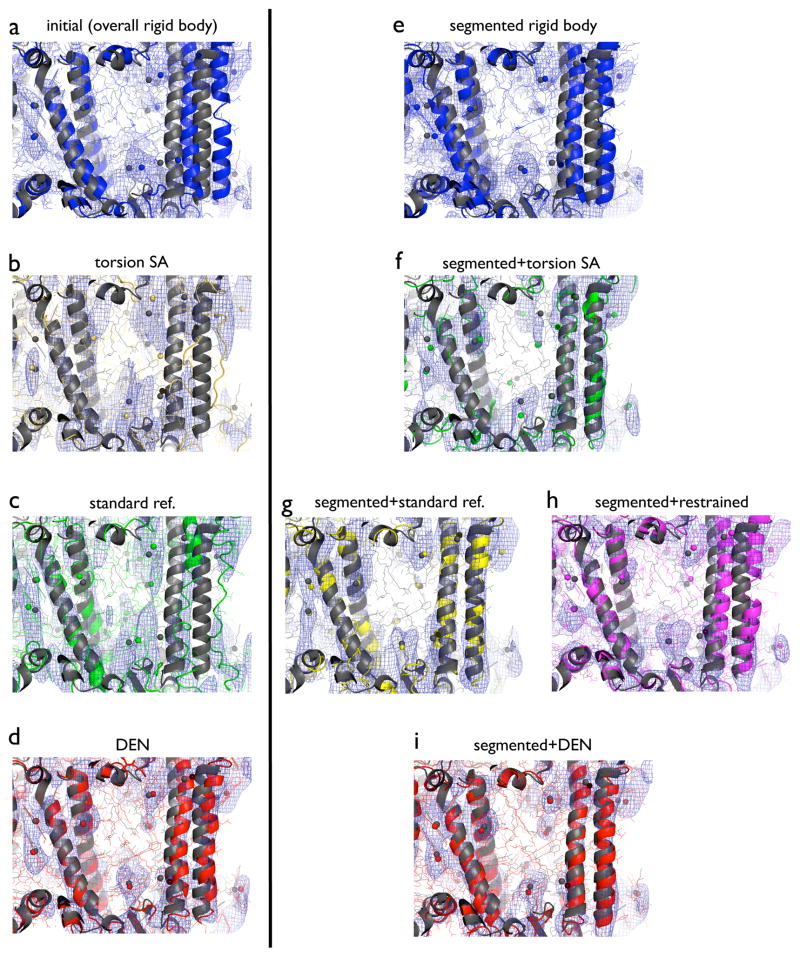
Figure 2.
Models and corresponding m2Fo-DFc electron density maps for specified refinements against the 7.4 Å diffraction data of PSI, starting from model M6. The electron density maps (blue mesh) were calculated with phases from the corresponding refined model and contoured at 1.5 σ. The 2.5 Å structure of PSI (PDB ID 1jb0) is shown in dark gray in each of the panels. Spheres indicate Mg2+ ions at the center of the chlorin rings. All non-hydrogen atoms are shown (lines) along with a cartoon representation. The region shown in the figure includes four α-helices (residues 54–100, 155–181, 669–694, and 720–750 of chain A) along with their protein environment and associated co-factors. (a) Initial, overall rigid-body refined model (blue). (b) Model obtained by torsion angle simulated annealing (yellow). (c) Model obtained by standard refinement (green). (d) Model obtained by DEN refinement (red). (e) Model obtained by segmented rigid-body refinement (blue). (f) Model obtained by torsion angle simulated annealing with initial segmented rigid-body refinement (green). (g) Model obtained by standard refinement with initial segmented rigid-body refinement (yellow). (h) Model obtained by refinement with secondary structure and reference restraints with phenix.refine with initial segmented rigid-body refinement (magenta). (i) Model obtained by DEN refinement with initial segmented rigid-body refinement (red). Refinement protocols are described in Experimental Procedures. See also Figure S2.