Figure 2.
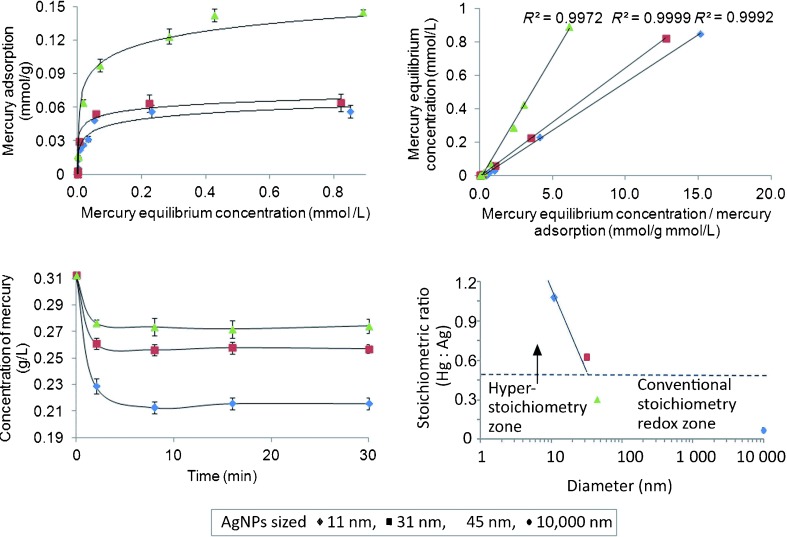
a) HgII adsorption isotherms on silica with AgNPs were performed in batch with 0.1 g of the three silver-containing silica samples per 40 mL of Hg(NO3)2 solution with concentrations ranging from 0.15 to 312 mg L−1 in the pH range of 4 to 7. The time allowed for establishing equilibrium was 120 min with constant shaking. The solution was separated by filtration and the residual mercury concentration was determined using inductively coupled plasma mass spectrometry (ICP-MS). b) Adsorption isotherms for the maximum adsorption of mercury onto different sized and quantities of AgNPs on silica were fitted to a Langmuir adsorption equation with R2 values above 0.99. c) The kinetics of HgII uptake, starting with a 312 mg L−1 stock solution, was studied for three different AgNP sizes on silica over 30 min. d) Ag rods, 0.01 mm in diameter and 5 mm in length, exhibited removal of HgII with a stoichiometric ratio of HgII to Ag0 below 0.5, but when using AgNPs this ratio increases from 0.29:1 up to the maximum of 1.125:1 when the Ag particle size is reduced from 45 nm to 11 nm. The change from conventional to hyper-stoichiometry occurs around 32 nm.
