Figure 7.
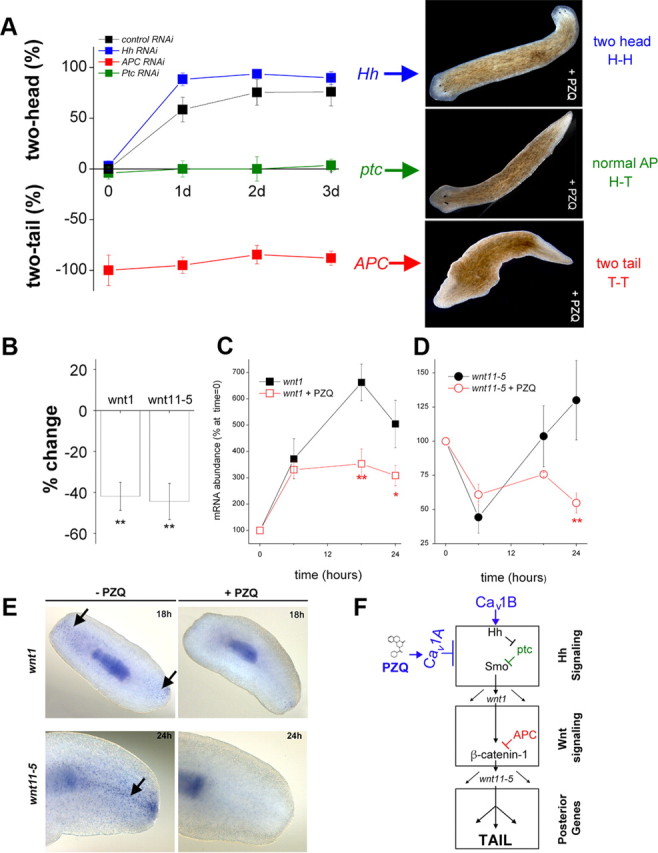
Inhibitory interaction of PZQ-evoked Ca2+ influx with Hh/Wnt signaling pathway. A, Left, Chemical genetic screen of PZQ efficacy in different cohorts of RNAi worms, including negative RNAi control (black), Hh (blue), APC (red), and Ptc (green) RNAi. The duration of PZQ exposure (90 μm) is shown on the abscissa, and the resulting bipolarity (two-headed, normal, two-tailed) is shown on the ordinate. Right, Representative images of dominant phenotype for Hh, Ptc, and APC RNAi worms exposed to PZQ. B, qPCR data of changes in wnt1 and wnt11-5 levels in regenerating trunk fragments exposed to PZQ (90 μm, 24 h) relative to untreated controls. C, qPCR analysis of wnt1 mRNA levels in the posterior blastema at indicated times after amputation (at t = 0) in the absence (black) and presence of PZQ (red squares, 90 μm). Asterisks indicate probability of similarity at p < 0.05 (*) and p < 0.01 (**). D, Similar qPCR analysis for wnt11-5 levels. E, In situ hybridization of wnt1 and wnt11-5 (arrowed) in the absence and presence of PZQ (90 μm) in regenerating trunk fragments at indicated times. F, Schematic of signaling modules involved in anterior–posterior specification. At least two distinct signal transduction pathways—Hedgehog (top) and Wnt signaling (middle) modules—control AP specification during regeneration as evidenced by RNAi of individual components of each module. These modules culminate to impact levels of βcatenin-1, which regulate a posterior fate circuit. Our data demonstrate an interaction of PZQ-evoked Ca2+ influx via Cav1A with Hh/Wnt signaling (top), localized upstream to APC within the Hh signaling module. Cav1B likely inhibits the trafficking/release of Hh from neurons (see Discussion). GenBank accession numbers: APC, HQ738520; βcatenin-1, HQ738521; wnt11-5, HQ738522; wnt1, HQ738523.
