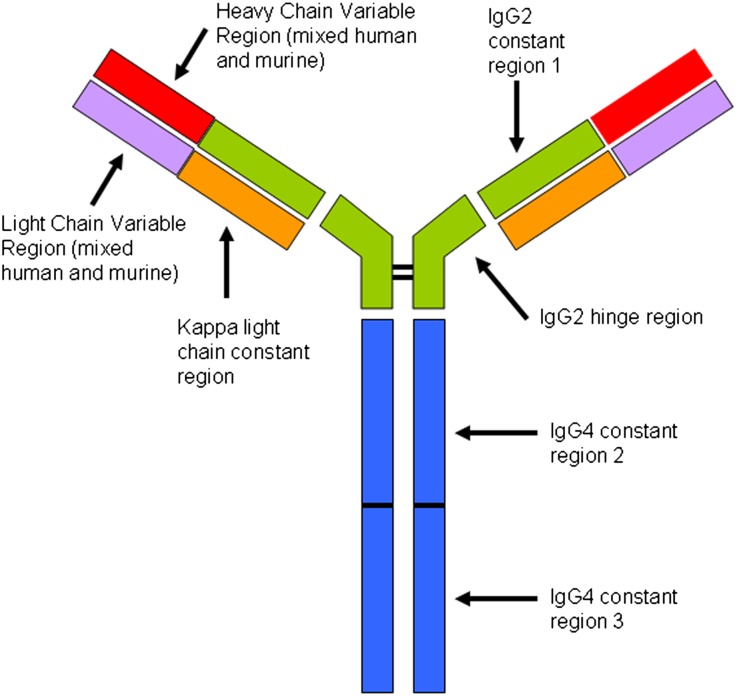
Figure 6.
Structural features of eculizumab. Adapted from Rother et.al.15 Constant regions CH2 and CH3 from IgG4 are fused to the IgG2 hinge region and CH1 domain and then paired with a κ light chain. The variable regions of the light and heavy chains are composed of the murine-derived sequences that have high affinity for C5, admixed with human germline framework regions. The result is a hybrid Ig with high affinity for C5 but without the ability to activate complement or bind Fc receptors. Post-treatment biopsies show evidence of eculizumab binding to glomeruli, TBMs, and vessel walls in the form of immunofluorescence staining for IgG2, IgG4, and κ light chain, with negative staining for IgG1, IgG3, and λ light chain.