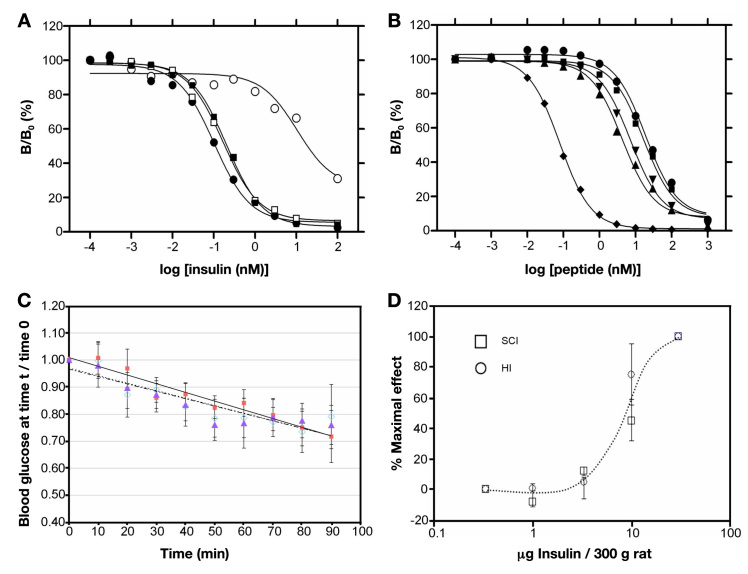
Figure 6.
Receptor-binding studies and biological activity in rats. (A) Stability of the receptor-binding activity of SCI-57 and wild-type insulin on prolonged gentle agitation at 37 °C. The following representative competitive-displacement curves are shown: assays conducted at the start of the incubation (SCI-57 ▪; insulin •), assay performed after 42 days of agitation (HI ∘), or assay performed after 90 days (SCI-57 ▫). Results are expressed as the ratio (B/B0) of 125I-[TyrA14]-insulin specifically bound at a given concentration of cold insulin relative to bound 125I-[TyrA14]-insulin specifically bound in absence of competing unlabeled insulin. (B)Competitive binding of insulin analogs to IGF-1R: SCI-57 (▪), wild-type insulin (●), [HisA8, AspB10, AspB28, ProB29]-insulin (▲), AspB10-insulin (▼), and human IGF-I (♦). (C) Decrease in blood glucose concentration with time (in minutes): regular human insulin (◊), the 57-residue single-chain analog (SCI; ▪), and its two-chain derivative lacking the linker (▲). Initial rates and maximal extents of glucose-lowering activity were indistinguishable. (D) A log plot of concentration-dependent efficacy in the rat shows no difference relative to wild-type insulin.