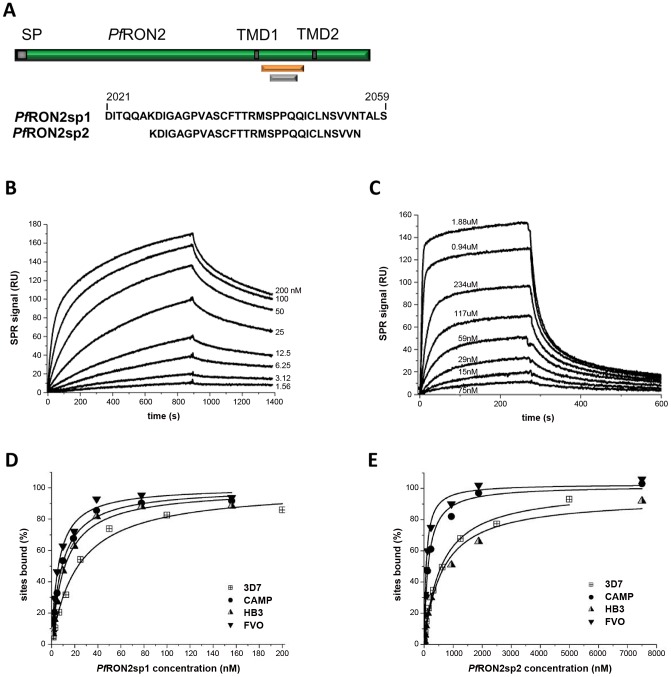
Figure 1. Surface Plasmon Resonance studies of peptides PfRON2sp1 and PfRON2sp2 binding to recombinant PfAMA1 from multiple strains reveal that PfRON2sp1 has a consistently higher affinity.
(A) PfRON2sp1 (orange) and PfRON2sp2 (grey) represent peptides of PfRON2 (green). SP, signal peptide. TMD, putative transmembrane domain. (B). Sensorgrams showing PfRON2sp1 (analyte) binding to PfAMA1 3D7 (immobilized). The PfRON2sp1 concentrations are indicated for each curve (nM). (C). Sensorgrams showing PfRON2sp2 (analyte) binding to PfAMA1 CAMP (immobilized), with PfRON2sp2 concentrations indicated. (D, E). Variation percentage of bound sites (deduced from the steady-state response) with respect to analyte concentration (D, PfRON2sp1; E, PfRON2sp2) obtained from binding to immobilized recombinant PfAMA1 from strains 3D7 (shown in B), CAMP (shown in C), FVO and HB3. The derived apparent equilibrium dissociation constants KD are given in Table 1.