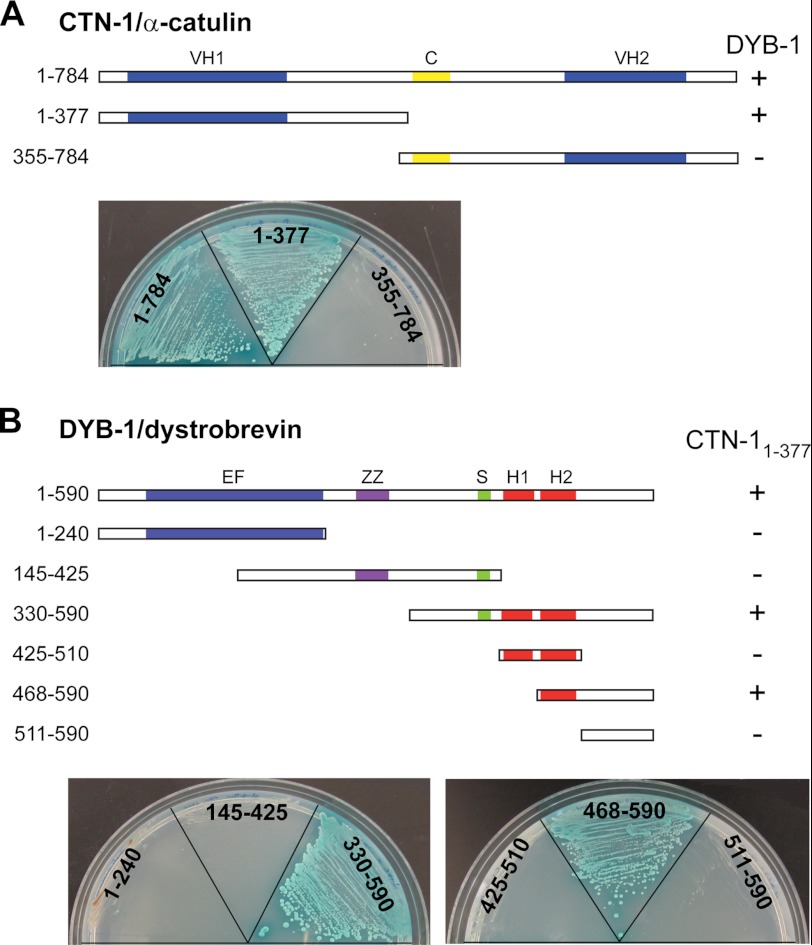
FIGURE 2.
Identification of interaction between DYB-1/dystrobrevin and CTN-1/α-catulin using yeast two-hybrid analysis. A, VH domain 1 (amino acids 1–377) of CTN-1 interacts with DYB-1, whereas the coiled-coil (C) and VH2 domains (amino acids 355–784) do not. The full-length and truncated CTN-1 cDNA sequences were fused with the DNA binding domain of GAL4 transcription factor, whereas the DYB-1 full-length cDNA sequence was fused with the activation domain. These constructs were transformed into two haploid yeast strains, and the resulting strains were mated with each other. Diploids showing a positive interaction were selected on quadruple dropout plates (−histidine, −alanine, −leucine, −tryptophan) containing X-α-Gal. + indicates the diploid yeast colonies that grow on selective plates and display blue color, whereas − indicates the diploid yeast that cannot grow on selective plates. B, various constructs of DYB-1 were tested for a positive interaction with the VH1 domain of CTN-1. The C-terminal region of DYB-1 was found to interact with CTN-1. EF, EF hand; ZZ, ZZ-type zinc finger domain; S, syntrophin binding domain; H1 and H2, coiled-coil motifs.