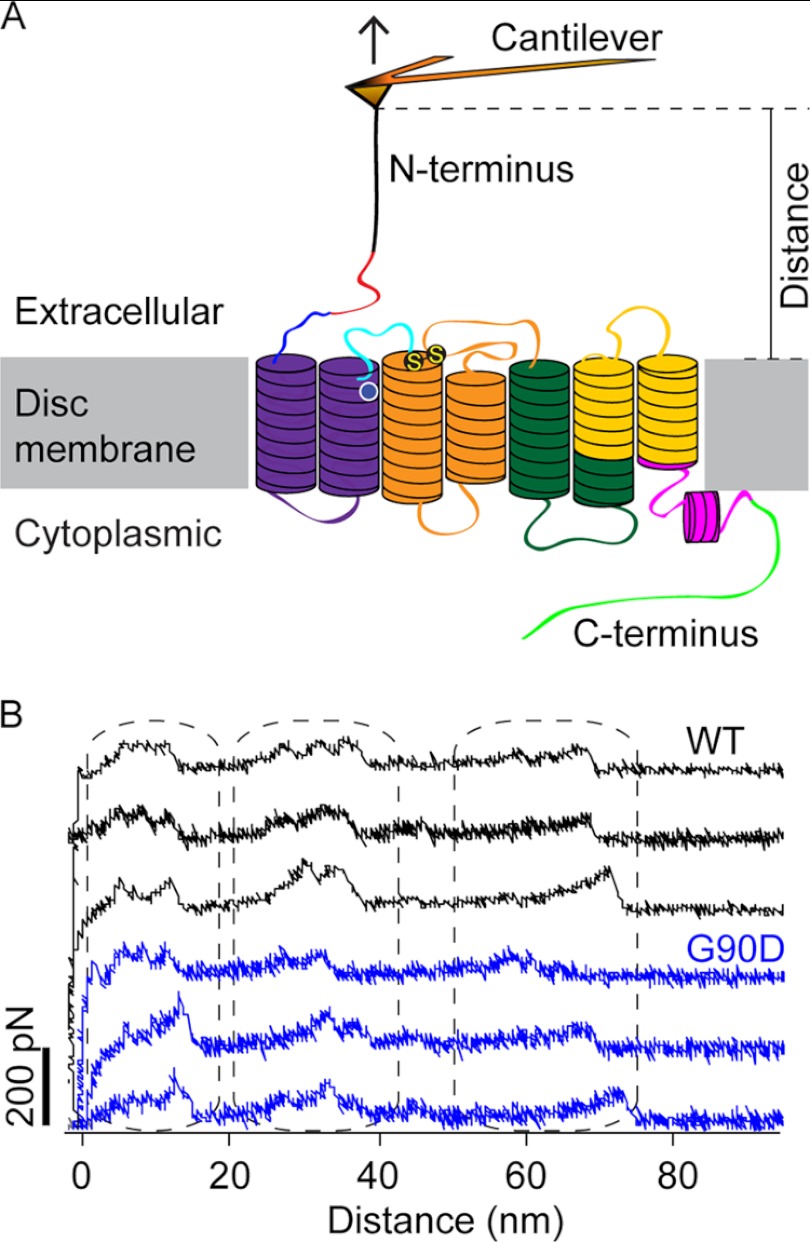
FIGURE 2.
SMFS of rhodopsin. A, illustration of SMFS on rhodopsin embedded in ROS disc membranes. In SMFS, the AFM cantilever tip is brought in contact with the N-terminal end of rhodopsin and then retracted from the membrane. This retraction applies mechanical stress and induces stepwise unfolding of rhodopsin. For each attempt, force and tip sample distances are recorded in F-D curves (as shown in B). The location of the G90D mutation is highlighted (white circle), and S-S denotes the conserved disulfide bond between residues Cys-110 and Cys-187. B, examples of F-D curves obtained from the mechanical unfolding of single wild-type (black) or G90D mutant (blue) rhodopsin. Each force peak of a F-D curve records the rupture of bonds and the subsequent unfolding of a stabilizing structural segment. The distance at which a force peak was detected localizes the molecular interaction that stabilized the structural segment.