Figure 7. An anion channel mediates LiGluR-evoked glutamate release by astrocytes.
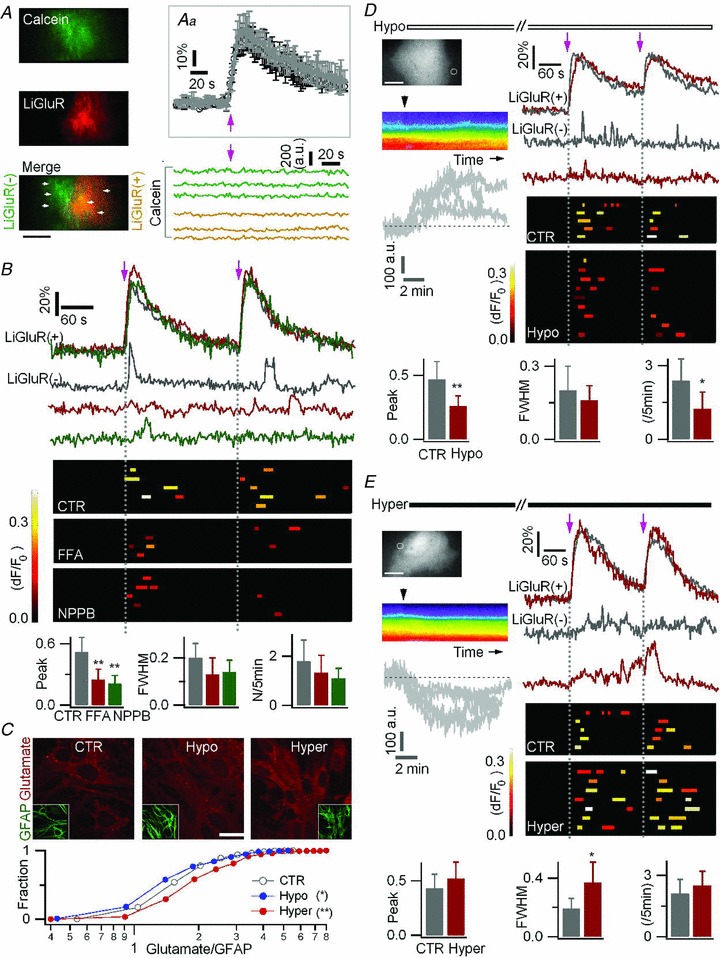
A, astrocytes were co-transfected with LiGluR-RFP and AsRed, and loaded with calcein AM. Inset (Aa), similar LiGluR-evoked Ca2+ rises in LiGluR(+) astrocytes in the absence (average black trace, n = 6) and in the presence of calcein (average grey trace, n = 6). Bottom, LiGluR photoactivation (arrow) left the calcein fluorescence stable in the ROIs (left panel, arrowhead, 2 μm × 2 μm) in LiGluR(+) cells (green traces) and LiGluR(−) cells (yellow traces). Bar, 5 μm. B, astrocytes were co-transfected with LiGluR-RFP and AsRed, and loaded with Fluo-4 AM. The LiGluR-evoked Ca2+ transients in LiGluR(−) astrocytes were reduced by the anion channel blockers, flufenamic acid (FFA, 50 μm, red) and 5-nitro-2-(3-phenylpropylamino) benzoic acid (NPPB, 100 μm, green). Control (CTR, grey traces, 12 events, 5 cells). FFA (red traces, 7 events, 4 cells). NPPB (green traces, 7 events, 5 cells. C, astrocytes were immunostained with glutamate (red) and GFAP (green) antibodies, and the glutamate/GFAP fluorescence intensity ratio was plotted as a cumulative histogram. Intracellular glutamate immunostaining was altered by 20 min treatment of hypo- (blue) or hyper-tonic solutions (red) (n = 244–606 regions from 3 trials for each condition, KS test). Bar, 10 μm. D–E, effect of hypo-osmotic (−25 mm NaCl) and hyper-osmotic (+50 mm sucrose; 15–20 min) solutions on LiGluR-evoked Ca2+ transients in LiGluR(−) astrocytes co-transfected with LiGluR-RFP and AsRed, and loaded with Fluo-4 AM. Left, pseudocolour kymographs show the transient cell swelling or shrinking, respectively, following the change of osmotic pressure (black arrowhead, application time) on GFP-expressing astrocytes. The same result is obtained when plotting the fluorescence change in ROIs lining the outer/inner side of the cell border (grey traces, n = 4 cells each). Right, sample traces and summary plots of LiGluR-evoked Ca2+ transients in LiGluR(−) astrocytes. Bottom, statistical comparison of LiGluR-evoked Ca2+ transients in control (CTR, 14 events, 5 cells), hypotonic (Hypo, 16 events, 9 cells), and hypertonic (Hyper, 23 events, 7 cells) solutions. Bars, 10 μm.
