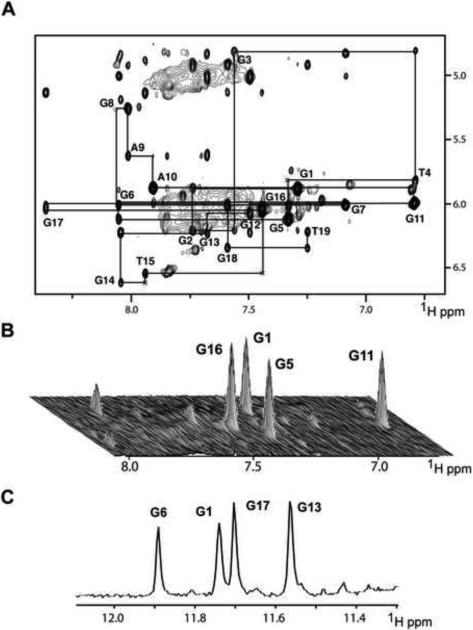
Figure 2. Non-exchangeable Proton Assignments of chl1 Intronic 19-mer Sequence in 50 mM K+, 5 mM phosphate-2H2O buffer, pH 6.8, at 25°C.
(A) Expanded NOESY contour plot (200 ms mixing time) correlating base and sugar H1' protons. The line connectivities trace NOEs between a base proton (H8 or H6) and its own and 5'-flanking sugar H1' protons. Intraresidue base to sugar H1' NOEs are labeled with residue numbers. (B) Stacked plot of short mixing time (50 ms) NOESY data set under same buffer conditions as in (A). The strong intraresidue guanosine H8-H1' cross-peaks (syn glycosidic bonds) are labeled and can be distinguished from weak cross-peaks (anti glycosidic bonds). (C) Imino proton NMR spectrum of chl1 intronic sequence recorded after 45 min following transfer after lyophilization from H2O to 2H2O solution. Assignments of slowly exchanging imino protons are listed over the spectrum.