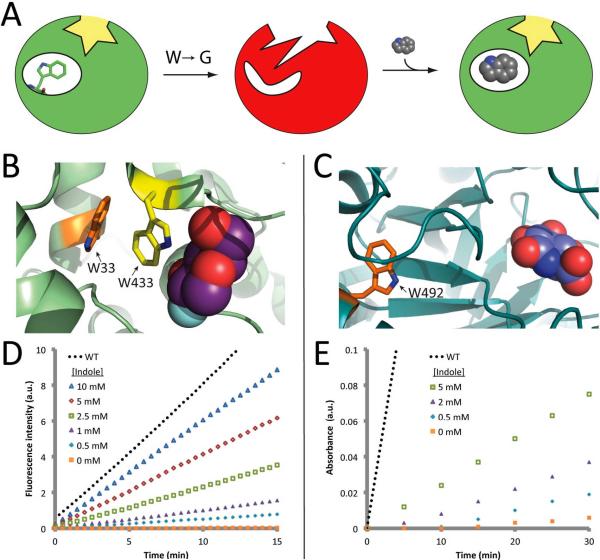
Figure 1. Indole rescue.
(A) Mutation of a remote “buttressing” tryptophan leads to structural disruption of catalytic geometry, resulting in loss of activity. Subsequent addition of exogenous indole restores structure, and thus activity. (B) Crystal structure of β-gly with a substrate analog (2-deoxy-2-fluoro-glucose, purple spheres)16, showing the “buttressing” tryptophan (Trp33, orange) and a nearby tryptophan (Trp433, yellow). (C) Crystal structure of β-gluc17 with a substrate analog (glucaro-δ-lactam, blue spheres) showing the “buttressing” tryptophan (Trp492, orange). (D) Indole-dependent activity of β-gly W33G, measured spectrofluorometrically using 100 μM fluorescein di-β-D-galactopyranoside (FDG) as a substrate. (E) Indole-dependent activity of β-gluc W492G, measured spectrophotometrically using 40 mM 2-nitrophenyl-β-D-glucopyranoside (ONPGlc) as a substrate. No indole was included in data for either wild-type enzyme shown here.