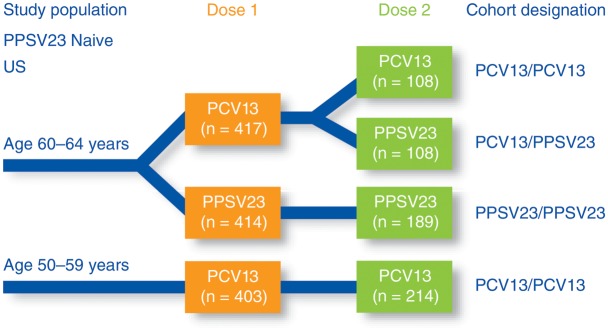
Figure 1.
Design of a phase 3, randomized, modified double-blind, active-controlled, multicenter trial (Study 004) conducted in the United States in subjects naive to 23-valent pneumococcal polysaccharide vaccine (PPSV23). Subjects aged 60–64 years were randomized to receive either 13-valent pneumococcal conjugate vaccine (PCV13) or PPSV23 as a first dose; subjects aged 50–59 years received PCV13 only as a first dose. All groups received a second vaccination with either PCV13 or PPSV23, 3–4 years after the first dose. Opsonophagocytic activity was measured at the time of the first dose, 1 and 12 months after the first dose, at the time of the second dose, and 1 month after the second dose. Abbreviations: PCV13, 13-valent pneumococcal conjugate vaccine; PPSV23, 23-valent pneumococcal polysaccharide vaccine.