Abstract
The apolipoprotein E ε4 gene is the most important genetic risk factor for sporadic Alzheimer’s disease, but the link between this gene and neurodegeneration remains unclear. Using array tomography, we analysed >50 000 synapses in brains of 11 patients with Alzheimer’s disease and five non-demented control subjects and found that synapse loss around senile plaques in Alzheimer’s disease correlates with the burden of oligomeric amyloid-β in the neuropil and that this synaptotoxic oligomerized peptide is present at a subset of synapses. Further analysis reveals apolipoprotein E ε4 patients with Alzheimer’s disease have significantly higher oligomeric amyloid-β burden and exacerbated synapse loss around plaques compared with apolipoprotein E ε3 patients. Apolipoprotein E4 protein colocalizes with oligomeric amyloid-β and enhances synaptic localization of oligomeric amyloid-β by >5-fold. Biochemical characterization shows that the amyloid-β enriched at synapses by apolipoprotein E4 includes sodium dodecyl sulphate-stable dimers and trimers. In mouse primary neuronal culture, lipidated apolipoprotein E4 enhances oligomeric amyloid-β association with synapses via a mechanism involving apolipoprotein E receptors. Together, these data suggest that apolipoprotein E4 is a co-factor that enhances the toxicity of oligomeric amyloid-β both by increasing its levels and directing it to synapses, providing a link between apolipoprotein E ε4 genotype and synapse loss, a major correlate of cognitive decline in Alzheimer’s disease.
Keywords: Alzheimer's disease, apolipoprotein E, synapse, array tomography, oligomeric amyloid-β
Introduction
Inheritance of the apolipoprotein E (APOE) ε4 allele is, after age, the single most potent and common risk factor for late onset Alzheimer’s disease. APOE ε4/4 increases risk more than ∼12-fold, and APOE ε3/4 about 3-fold, compared with the APOE ε3/3 genotype (Roses, 1996). Inheritance of APOE ε4 also leads to a markedly earlier age of onset (Corder et al., 1993). Synapse loss is a strong correlate of cognitive decline in Alzheimer’s disease (DeKosky and Scheff, 1990) and imaging studies have suggested the occurrence of synaptic dysfunction decades before Alzheimer’s disease onset in APOE ε4 patients (Reiman et al., 1996). However, the mechanistic link between apoE isoform and these effects in Alzheimer’s disease remains unknown.
The amyloid hypothesis of Alzheimer’s disease suggests that pathogenesis is initiated by amyloid deposition. Initially envisioned as senile plaques, a more recent revision of the hypothesis suggests that soluble oligomeric forms of amyloid-β are a critical mediator of amyloid-β neurotoxicity (McLean et al., 1999; Walsh et al., 2002; Lacor et al., 2007; Koffie et al., 2009, 2011; Tomic et al., 2009). In particular, SDS-stable dimers of amyloid-β isolated from human brain have been shown to adversely affect synaptic plasticity in slice culture (Shankar et al., 2008; Mc Donald et al., 2010) and intact brains (Cleary et al., 2005). Although the exact relationship between fibrillar deposits of amyloid-β and oligomeric amyloid-β is still to be refined, data from amyloid precursor protein overexpressing transgenic mouse models suggest that they exist in equilibrium, in that recent studies using oligomer-specific antibodies revealed a ‘halo’ of oligomeric amyloid-β surrounding plaques (Koffie et al., 2009; Gaspar et al., 2010). Oligomeric amyloid-β puncta near plaques often co-localized with PSD95-positive puncta, corresponding to a zone of diminished synaptic density (Koffie et al., 2009). However, whether or not oligomeric amyloid-β plays a role in synaptic dysfunction in human Alzheimer’s disease is controversial. We now demonstrate that oligomeric amyloid-β is elevated specifically at synaptic sites in human Alzheimer’s brains, supporting earlier animal studies. Moreover, we show that the apoE4 isoform increases synapse-associated oligomeric amyloid-β compared with apoE3 in human brains with Alzheimer’s disease.
To test the hypothesis that the apoE4 isoform acts in concert with oligomeric amyloid-β to accelerate synaptotoxic effects of oligomeric amyloid-β, we extended the array tomography technique previously used in experimental animals (Micheva and Smith, 2007; Micheva et al., 2010) to fresh human brain autopsy material. We demonstrate that (i) a zone of increased oligomeric amyloid-β and decreased synaptic elements exists around plaques in human Alzheimer’s disease; (ii) a subset of pre- and postsynaptic puncta contain oligomeric amyloid-β; (iii) apoE is present at synapses in control and Alzheimer’s disease brains. In Alzheimer’s disease, oligomeric amyloid-β co-localizes with apoE at synapses in a genotype-dependent fashion E4>E3; (iv) there are higher levels of oligomeric amyloid-β and greater synapse loss around plaques in APOE ε4 cases compared with APOE ε3 cases; (v) biochemical isolation of synaptoneurosomes from APOE ε3/3 or APOE ε4/4 patients with Alzheimer’s disease confirm markedly elevated levels of oligomeric amyloid-β in the latter and show the presence of amyloid-β dimers and trimers; and (vi) in vitro experiments, using primary neurons exposed to oligomeric amyloid-β in the presence of lipidated apoE3 or apoE4 demonstrate apoE4>apoE3 mediated targeting of oligomeric amyloid-β to synaptic elements. Taken together, these data support the hypothesis that a major biological effect of apoE4 in Alzheimer’s disease is to enhance synaptic toxicity of oligomeric amyloid-β.
Materials and methods
Subjects
Brains from human subjects with a diagnosis of Alzheimer’s disease or no cognitive deficits were obtained through the Massachusetts Alzheimer’s Disease Research Centre and Massachusetts General Hospital Neuropathology department. All donor tissue was obtained in accord with local and National Institutional Review Board regulations. Characteristics of each subject (for array tomography: 11 subjects with Alzheimer’s disease, five controls; for biochemical synaptoneurosome experiments: 12 subjects with Alzheimer’s disease, six controls) are shown in Supplementary Tables 1 and 2. In synaptoneurosome experiments, subjects with Alzheimer’s disease with APOE ε3/3 versus APOE ε4/4 genotype were chosen from a previously studied large cohort (Serrano-Pozo et al., 2010) and matched for plaque burden (no statistical difference in burden between 3/3 and 4/4 cases). Plaque burden was determined as previously reported (Serrano-Pozo et al., 2010). Briefly, paraffin embedded tissue from the frontal cortex contralateral to that used in biochemical fractionation experiments was sectioned, stained with anti-amyloid β antibody 10D5 and the percentage of the total area stained was detected on an upright Leica microscope coupled with the BIOQUANT Nova Prime Software (MBSR).
Array tomography
Samples from the superior temporal gyri of each subject were collected and prepared into resin blocks as outlined elsewhere (Micheva and Smith, 2007; Koffie et al., 2009; Micheva et al., 2010); detailed protocols can be found in the Supplementary material. Ribbons of 70-nm sections were immunostained with primary antibodies at a 1:10 dilution in blocking buffer (mouse anti-amyloid-β oligomers; NAB61, courtesy V. Lee; Lee et al., 2006), R1282 (courtesy Dennis Selkoe), goat anti-PSD95 (Abcam Ab12093), rabbit anti-synapsin I (Millipore AB1543), goat polyclonal anti-apoE (Millipore AB947) or mouse anti-apoE antibody (WU E-4, from D. Holtzman). Secondary antibodies were diluted 1:50 in blocking buffer (donkey anti-rabbit Alexa Fluor® 488, Invitrogen; donkey anti-mouse Cy5, Jackson ImmunoResearch; donkey anti-goat Cy3, Jackson ImmunoResearch). Images were obtained from 7 to 30 serial sections using a Zeiss Axioplan LSM510 confocal/multiphoton microscope (×63 1.2 NA Plan Apochromatic water objective).
Images were analysed with ImageJ (National Institutes of Health open software) and MATLAB (Mathworks). Images from serial sections were stacked, aligned, cropped into volumes at known distances from plaques or phantom plaques, and an automated threshold-based program detected puncta and generated counts, volume of each puncta and an output image [courtesy of Brad Busse and P. Thevenaz et al. (1998)]. The output image stack from each channel of staining was imported into MATLAB for analysis of co-localization between stained proteins and to assess the distances between pre- and postsynaptic puncta. Image crops were processed in a semi-automated fashion blind to diagnosis, APOE genotype and distance from plaque.
Apolipoprotein E genotyping
ApoE genotype was determined with a Macherey-Nagel Kit using the manufacturer’s protocol. Purified DNA samples were amplified through PCR using forward primer 5′-taagcttggcacggctgtccaagg-3′ and reverse primer 5′-acagaattcgccccggcctggtacactgcc-3′. After PCR, amplified DNA was digested with HhaI restriction fragment endonuclease and electrophoresis performed on the resulting DNA fragments.
Biochemical fractionation experiments
Synaptoneurosome preparations were based on procedures described by Hollingsworth et al. (1985) with minor modifications. Briefly, 250 mg of frozen tissue (grey matter) was taken from the frontal cortex of each subject. The brain tissue was homogenized in 1.5 ml cold Buffer A (25 mM Tris, pH 7.5, 120 mM NaCl, 5 mM KCl, 1 mM MgCl2, 2 mM CaCl2, 1 mM dithiothreitol, protease inhibitors) using a Teflon-glass mechanical tissue grinder and filtered through 80-µm pore filters. A small portion of the filtrate was supplemented with 1% SDS, boiled for 5 min and centrifuged at 15 000g for 15 min, and the supernatant was collected as total extract. The other portion was further filtered through 5 -µm pore filters and centrifuged at 1000g for 10 min to pellet synaptoneurosomes. The supernatant was collected as cytosolic extract, which was further centrifuged at 100 000g for 20 min to remove microsomes. The synaptoneurosome pellet was washed once with cold Buffer A and centrifuged again at 1000g for 10 min. The pellet was extracted with 0.5 ml Buffer B (50 mM Tris pH 7.5, 1% SDS, 2 mM dithiothreitol) and boiled for 5 min. After centrifugation at 15 000g for 15 min, the supernatant was collected as synaptoneurosomal extract. All fractions were investigated using SDS-PAGE and western blotting with antibodies 82E1 (anti-amyloid-β, IBL), 3H1 (anti-apoE, Ottowa Heart Institute), ApoAI (Ottowa Heart Institute), PSD95 (Cell Signaling) and synaptophysin (Abcam). To confirm the localization of oligomeric amyloid-β in both the pre- and postsynaptic compartments, we also developed a new method of spreading biochemically isolated synaptoneurosome preparations on a slide, immunostaining them and imaging with fluorescence and brightfield microscopy. Antibodies used for this study were apoE (goat, Millipore AB947), vGlut1 (guinea pig, Millipore AB5905), MAP2 (chicken, Abcam AB5392) and NAB61 (mouse, made by V. Lee).
Cell culture experiments
Lipidated apoE particles were purified from human apoE2, apoE3 or apoE4 overexpressing immortalized astrocytes using an affinity column as described elsewhere (Morikawa et al., 2005) and in the Supplementary material. Primary neuronal cell cultures were prepared from cortical neurons from mice at embryonic Day 16. After growing for 1 week, cells were treated with culture media from Tg2576 neuronal culture, which contains naturally secreted oligomeric amyloid-β (0.5 ng/ml; Wu et al., 2010) and purified lipidated apoE2, apoE3, apoE4 or PBS for 48 h (5 µg/ml apoE with or without RAP-D3 for 24 h). Cultured neurons were then fixed with 4% paraformaldehyde and permeabilized with 2% saponin in Tris-buffered saline and immunostained. To stain synaptic elements, oligomeric amyloid-β and apoE, fixed cultured neurons were immunostained with the same antibodies used for array tomography, imaged using a Zeiss Axioplan LSM510 confocal/multiphoton microscope and analysed using ImageJ (National Institute of Health open software) and MATLAB (MathWorks, Inc.).
Statistics
Normality of data was assessed using the Shapiro–Wilks test. For each case, the mean (for normally distributed data) or median (for non-normal data) of each measure was calculated. The means or medians of each case were then used to compare all cases in each group (for example comparing all control cases to all cases with Alzheimer’s disease). For normally distributed data, means were compared using ANOVA (for more than two groups) and t-tests (for two group comparisons). Non-normal data were compared by Wilcoxon tests (for comparisons of two groups) and Kruskal–Wallis tests (for comparisons of more than two groups; for example, distances from a plaque edge). Spearman’s rho correlations were used to determine correlations. Calculated comparisons were at confidence interval (CI) 95%; i.e. P < 0.05 was considered significant. In the text, normal data are presented as mean ± standard deviation and non-normal data are presented as median (minimum value–maximum value). Graphs are presented as means ± standard errors.
Results
Progressive synapse loss near plaques in human Alzheimer’s disease brains
Observing individual synapses is technically difficult in thick sections due to poor axial resolution of even confocal microscopy in relation to the size of these structures. Electron microscopy, which can resolve these structures, is challenging because post-mortem human brain is generally not adequate for electron microscopy and immunostaining of sections prepared for electron microscopy with glutaraldehyde can be problematic. Moreover, the number of synapses that can be interrogated by electron microscopy is limited. We utilized a new histological approach, array tomography, which was designed for high-resolution immunofluorescent characterization of synapses in the cortex of experimental animals (Micheva and Smith, 2007; Micheva et al., 2010). Like electron microscopy, the z-axis resolution of array tomography is ∼70 nm (compared with 1 µm for standard light microscopy), but unlike electron microscopy the tissue need not be glutaraldehyde fixed to retain ultrastructure. Moreover, array tomography lends itself to image analysis approaches that allow examination of tens of thousands of synapses, providing robust statistical power to examine the effect of Alzheimer’s disease pathology on synapse collapse. We used this technique to study synapses near plaques in amyloid precursor protein transgenic mice (Koffie et al., 2009) and now apply it to human Alzheimer’s disease samples to examine the relationship of oligomeric amyloid-β to plaques in human brains and to determine whether APOE genotype influences this relationship.
First, we assessed synapse loss around plaques in the cortical neuropil of layer II–III of superior temporal gyri of the brains of 11 patients with Alzheimer’s disease and five non-demented controls (Supplementary Table 1). Ribbons of 70-nm sections were immunostained for presynaptic elements (synapsin I), postsynaptic elements (PSD95) and oligomeric amyloid-β (NAB61; Lee et al., 2006). NAB61 is a conformer specific antibody that has been demonstrated to preferentially recognize toxic amyloid-β oligomers (Lee et al., 2006; Koffie et al., 2009). Image stacks were obtained from these ribbons and reconstructed into 3D image sets for analysis (Fig. 1A and B).
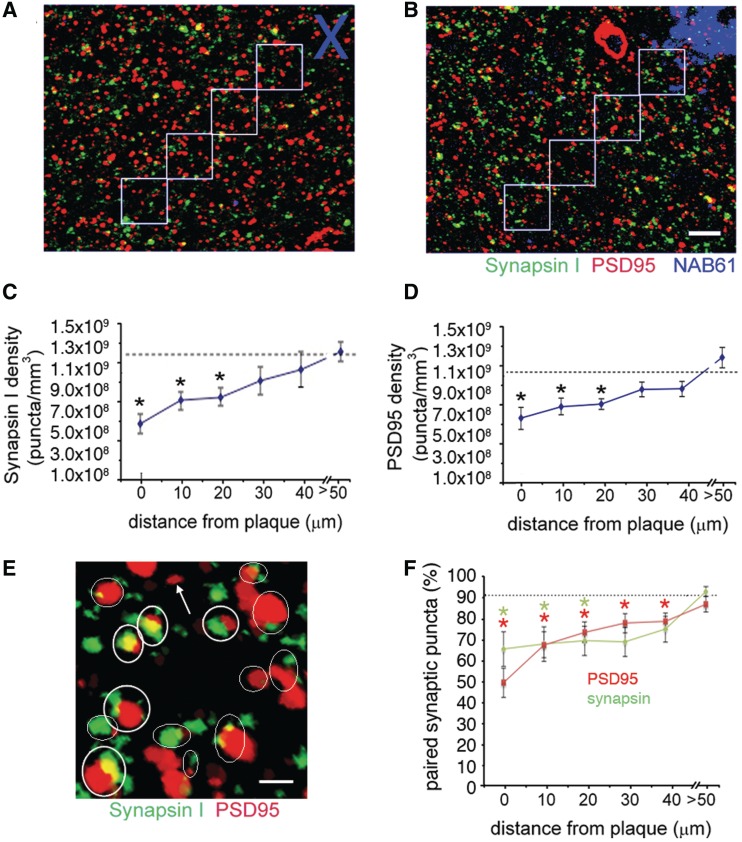
Figure 1.
Synapse density decreases approaching plaques in Alzheimer’s disease brain. Array tomography was used to obtain image stacks of immunostained pre- (synapsin I) and postsynaptic (PSD95) elements in control (A) and Alzheimer’s disease (B) brains at 10-µm increments from a plaque edge (phantom plaques in control represented by blue cross in A were chosen randomly) or at distances further than 50 µm from the nearest plaque. Quantification reveals a significant loss of both (C) pre- and (D) postsynaptic elements approaching plaques in Alzheimer’s disease brains compared with control levels (dotted lines show control averages; Kruskal–Wallis test, synapse density independent variable, plaque distance dependent variable, P < 0.05, post hoc Wilcoxon all pairs *P < 0.05). Pairing analysis was done to determine which synaptic puncta had a partner within 0.5 µm (examples circled in E). Quantification (F) shows that ∼90% of both postsynaptic density and synapsin puncta have a partner in control brain and >50 µm from plaques in Alzheimer’s disease brain, but that the pairing is significantly decreased near plaques in Alzheimer’s disease leaving ‘orphaned’ puncta (arrow) (Kruskal–Wallis test, pairing independent variable, plaque distance dependent variable, P < 0.05, post hoc Wilcoxon all pairs *P < 0.05). Images are maximum intensity z-projections of sections from array tomography ribbons (10 sections projected in A and B, 17 sections projected in E). Scale bars: B = 5 µm, E = 1 µm.
We examined 46 plaques and >50 000 synapses (for numbers of cases, plaques, and synapses, see Table 1) and found that in Alzheimer’s disease brains, synapse density is reduced near plaques (Kruskal–Wallis test, synapse density dependent variable, distance to nearest plaque independent variable, P = 0.001 for PSD95 density, P = 0.003 synapsin I density). In the immediate vicinity of plaques (within 10 µm of the edge of plaques) synapse density is drastically reduced by ∼65% compared with control values [median 3.4 × 108 (minimum value–maximum value 2.26 × 108–1.11 × 109) synapsin I puncta per mm3, 5.08 × 108 (1.20 × 108–1.20 × 109) PSD95 puncta per mm3 in Alzheimer’s cases near plaques compared with 1.2 × 109 (1.09 × 109–1.34 × 109) synapsin I puncta per mm3, 1.1 × 109 (0.97 × 109–1.28 × 109) PSD95 puncta per mm3 in controls; Wilcoxon each pair P < 0.01 for both synapsin and PSD95]. Further, both synapsin I density and PSD95 density correlate with distance from plaques, increasing linearly from the edge of plaques when examined in 10-µm increments to approach control levels at distances >50 µm away from plaques (Spearman’s rho correlation coefficients 0.41 for synapsin, 0.48 for PSD95, P < 0.01; Fig. 1A–D). These findings demonstrate that similar to mouse models of plaque deposition (Spires et al., 2005; Koffie et al., 2009), synapse density decreases progressively approaching plaques in human Alzheimer’s disease patient brains.
Table 1.
Numbers of samples from array studies
| Synapsin I, PSD95, NAB61 study |
ApoE, synapsin I, NAB61 study |
||||||||
|---|---|---|---|---|---|---|---|---|---|
| Diagnosis | APOE genotype | Cases | Plaques | Presynaptic puncta | Postsynaptic puncta | Oligomeric amyloid-β puncta | Presynaptic puncta | ApoE puncta | Oligomeric amyloid-β puncta |
| AD | 3/3 | 3 | 12 | 7256 | 7767 | 1070 | 2826 | 3597 | 177 |
| AD | 3/4 | 5 | 22 | 14 048 | 12 011 | 3165 | 11 138 | 9884 | 3049 |
| AD | 4/4 | 3 | 12 | 7394 | 5410 | 1406 | 1437 | 2401 | 2738 |
| control | 3/3 | 5 | 0 | 7490 | 6992 | 315 | 8247 | 12 084 | 187 |
AD = Alzheimer’s disease.
To investigate whether there are any pre- or postsynaptic elements left ‘orphaned’ without a synaptic partner (e.g. a postsynaptic density without a connected presynaptic bouton), we performed a pairing analysis to determine whether each synaptic puncta had a potential synaptic partner within 0.5 µm, close enough to form a synaptic connection. While without direct visualization of the postsynaptic density and synaptic boutons by electron microscopy, we cannot conclude that all of these ‘pairs’ are true synapses, this is a high-throughput estimate of whether there are isolated synapses that do not have a partner. This pairing analysis demonstrates that in control brain and far from plaques in Alzheimer’s disease cases, ∼90% of postsynaptic density puncta have a potential synapsin partner within 0.5 µm and conversely that 90% of synapsin puncta have a postsynaptic density partner within the same distance (Fig. 1E and F). Interestingly, approaching plaques, this pairing significantly decreases (Kruskal–Wallis P = 0.01 synapsin with partner, P = 0.003 PSD95 with partner), indicating synapses may not be lost in pairs, but rather that the pre- or postsynaptic density may degenerate first leaving an orphaned partner.
In mice, we previously observed that synapse loss around plaques is associated with elevated levels of oligomeric amyloid-β. The array data from human Alzheimer’s disease brains reveals a similar concentration of oligomeric amyloid-β in the vicinity of plaques with the highest levels immediately surrounding plaques (10% of the neuropil volume occupied by NAB61 staining) decreasing exponentially to control levels of <1% oligomeric amyloid-β burden at distances further than 50 µm from the plaque edge (Fig. 2A, Kruskal–Wallis test shows distance from plaque affects NAB61 burden P < 0.001, linear fit of log normal data shows exponential decay, r2 = 0.24, P < 0.001). NAB61 puncta vary in volume from below the resolution of the confocal microscope (calculated as 0.0016 µm3 based on detection of the Airy disk) to 1.39 µm3 (almost mini-plaques). In support of a role for oligomeric amyloid-β in synapse loss, synapse density correlated negatively with oligomeric amyloid-β burden (spearman’s rho correlation coefficient, −0.38 for PSD95, −0.48 for synapsin I; P < 0.01).
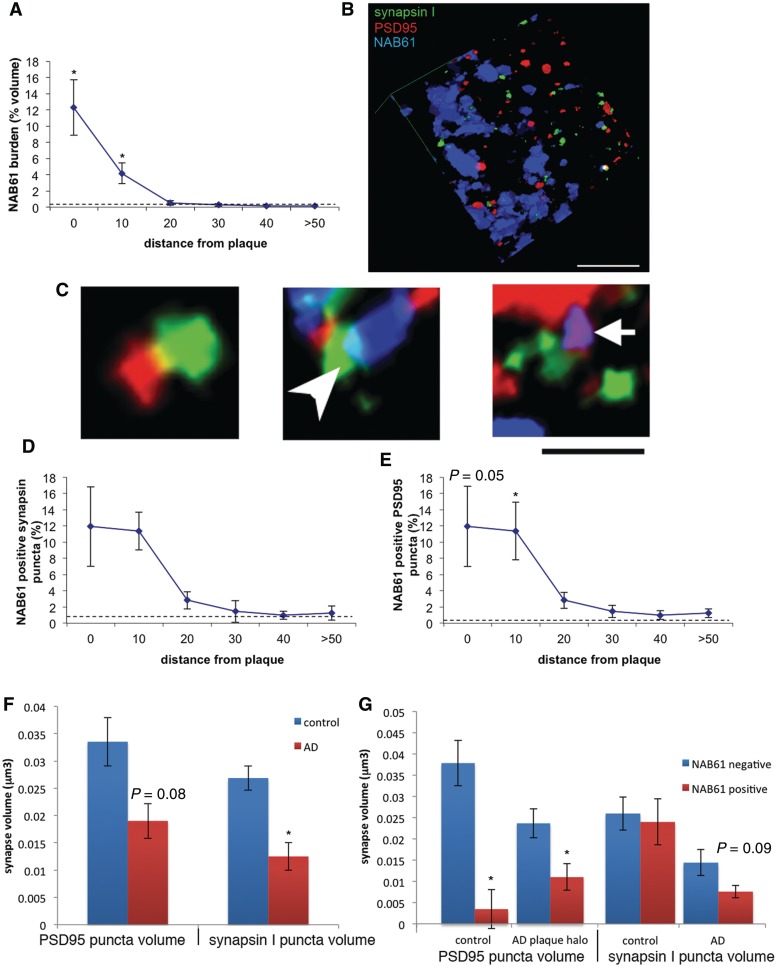
Figure 2.
Synapse loss correlates with oligomeric amyloid-β at synapses. The burden of oligomeric amyloid-β in Alzheimer’s disease brain is highest around plaques and decreases exponentially to control levels (represented by dotted line) with increasing distance from the plaque (A). Three dimensional reconstructions of array tomograms (12 sections reconstructed in B) reveal that oligomeric amyloid-β associates with a subset of synapses in Alzheimer’s disease brain. (C) Enlargements of the reconstruction showing examples of opposed pre- and postsynapses with no oligomeric amyloid-β (left), an oligomeric amyloid-β positive presynaptic element (arrowhead) and an oligomeric amyloid-β positive postsynaptic element (arrow). The level of co-localization between NAB61-positive puncta and presynaptic (D) or postsynaptic elements (E) is highest near plaques in Alzheimer’s disease and decreases exponentially to reach normal levels at distances >50 µm away from plaques (Kruskal–Wallis test with distance from plaque independent variable, P < 0.05, *P < 0.05 Wilcoxon all pairs post hoc test compared with control level). In Alzheimer’s disease brains, presysnaptic elements are shrunken compared with control brains (F, *P < 0.05 Wilcoxon test) and there is a trend towards shrinkage of postsynaptic densities (P = 0.08) that becomes significant when only the volumes within 10 µm of a plaque edge are considered. The presence of NAB61 positive oligomeric amyloid-β puncta at synapses is associated with smaller postsynaptic densities in this same region near plaques in Alzheimer’s disease brains and in control brains (G). Synapsin I puncta volumes are not significantly affected by the presence of oligomeric amyloid-β although there is a trend towards presynapse shrinkage in Alzheimer’s disease brains. Scale bars: B = 5 µm; C = 2 µm. AD = Alzheimer’s disease.
Three-dimensional reconstructions of array stacks allow precise quantification of the co-localization of oligomeric amyloid-β with synapses (Fig. 2B and C). At the edges of plaques, ∼12% of synaptic puncta are positive for oligomeric amyloid-β, decreasing exponentially to <2% at a distance >50 µm away from plaques (Fig. 2D and E, Kruskal–Wallis test shows distance to plaque significantly impacts the percentage of NAB61-positive synapses, P < 0.05 for per cent NAB61-positive PSD95 and per cent NAB61-positive synapsin, linear regressions of log data indicate exponential decay with r2 = 0.2, P < 0.01 for both per cent NAB61-positive postsynaptic density and per cent NAB61-positive synapsin). The extent of oligomeric amyloid-β co-localization with synaptic elements in Alzheimer’s disease brains in the zone around plaques was 90-fold higher than expected by chance (see Supplementary material for calculations). As expected, oligomeric amyloid-β is rare in control brains [0.02% (0–0.39%) of the neuropil occupied by NAB61 staining]. Nonetheless, despite the rarity of oligomeric amyloid-β puncta, they were ∼165-fold more likely than chance to associate with synaptic elements instead of random locations.
To test whether these observations are specific to the NAB61 antibody, we repeated the above experiments using another amyloid-β antibody (R1282), which recognizes all forms of amyloid-β including oligomers (Shankar et al., 2008). We confirmed that R1282-positive puncta associate with a subset of synapses, albeit the signal intensity of R1282 at synapses was weaker than NAB61 on array tomograms (Supplementary Fig. 1). These results suggest that soluble amyloid-β in a conformation recognized by both NAB61 and R1282 is indeed present at synapses in human Alzheimer’s disease brains and may have potent roles in inducing synaptic dysfunction.
There are significant differences between volumes of synaptic elements in control and Alzheimer’s disease brains (Fig. 2F). Overall, synapsin I puncta in Alzheimer’s disease brains are ∼50% smaller than those of controls [control median synapsin volume 0.028 µm3 (0.020–0.031µm3) Alzheimer’s disease case median 0.016 µm3 (0.001–0.026 µm3), P = 0.01 Wilcoxon test]. PSD95 puncta also show a trend towards being smaller in Alzheimer’s disease brains [control median PSD95 volume 0.036 µm3 (0.021–0.043 µm3), Alzheimer’s case median 0.026 µm3 (0.003–0.030 µm3), P = 0.08 Wilcoxon test]. Within 10 µm of a plaque edge, this shrinkage of PSD95 puncta in Alzheimer’s disease brains is significant [0.020 µm3 (0.004–0.059 µm3) in Alzheimer’s disease brain<10 µm from plaque, 0.036 µm3 (0.021–0.043 µm3) in control, P = 0.04, Wilcoxon each pair test]. In the same region where we observe postsynaptic density shrinkage in Alzheimer’s disease brain (within 10 µm of a plaque edge), we observe that NAB61-positive PSD95 puncta are 50% smaller than neighbouring synapses not contacted by oligomeric amyloid-β (P < 0.05 Wilcoxon test). The presence of oligomeric amyloid-β at presynaptic elements does not significantly impact volume, although there is a trend towards smaller synapsin I puncta in the presence of oligomeric amyloid-β in Alzheimer’s disease brain compared with synapsin I puncta without oligomeric amyloid-β (P = 0.09, Wilcoxon test). While there are very few synapses in control brains contacted by oligomeric amyloid-β, we still observe shrinkage of postsynaptic densities contacted by oligomeric amyloid-β puncta, indicating that the presence of oligomeric amyloid-β at the synapse may contribute to dendritic spine shrinkage (Fig. 2G).
Apolipoprotein E4 enhances accumulation of amyloid-β oligomers at synapses
The apoE protein decorates plaques in Alzheimer’s disease patients’ brains (Rebeck et al., 1993; Wisniewski et al., 1995; Jones et al., 2010) and inheritance of APOE ε4 has been implicated in the amount of fibrillar amyloid-β deposits observed in Alzheimer’s brains, age of dementia onset and synaptic dysfunction (Gomez-Isla et al., 1996; Reiman et al., 2005), but whether or not APOE ε4 impacts the levels of oligomeric amyloid-β or its association with synapses in the brain is unclear. We took advantage of the array tomography technology to further investigate the relationship of oligomeric amyloid-β and synapse density around plaques, in order to ask whether this was changed in patients with Alzheimer’s disease harbouring an APOE ε4 allele (ε4/4 or ε3/4 together called εx/4) compared with APOE ε3/3 patients. Analysis of array tomography data showed a genotype-specific difference in the extent to which synaptic loss had occurred: the extent of synaptic loss within 50 µm of plaques was highest for individuals harbouring one or two copies of APOE ε4, compared with APOE ε3/3 (Kruskal–Wallis test, effect of APOE genotype, P = 0.03, post hoc Wilcoxon each pair shows APOE ε3/3 versus APOE εx/4, P = 0.002, Fig. 3A). This decrease in synaptic density correlates with an APOE genotype specific effect on oligomeric amyloid-β burden. Oligomeric amyloid-β burden around plaques follows the trend E4>E3, declining exponentially in each group to reach control levels at distances >50 µm away from plaques (Fig. 3B, Kruskal–Wallis test effect of E4 P = 0.047, effect of plaque distance P < 0.0001).
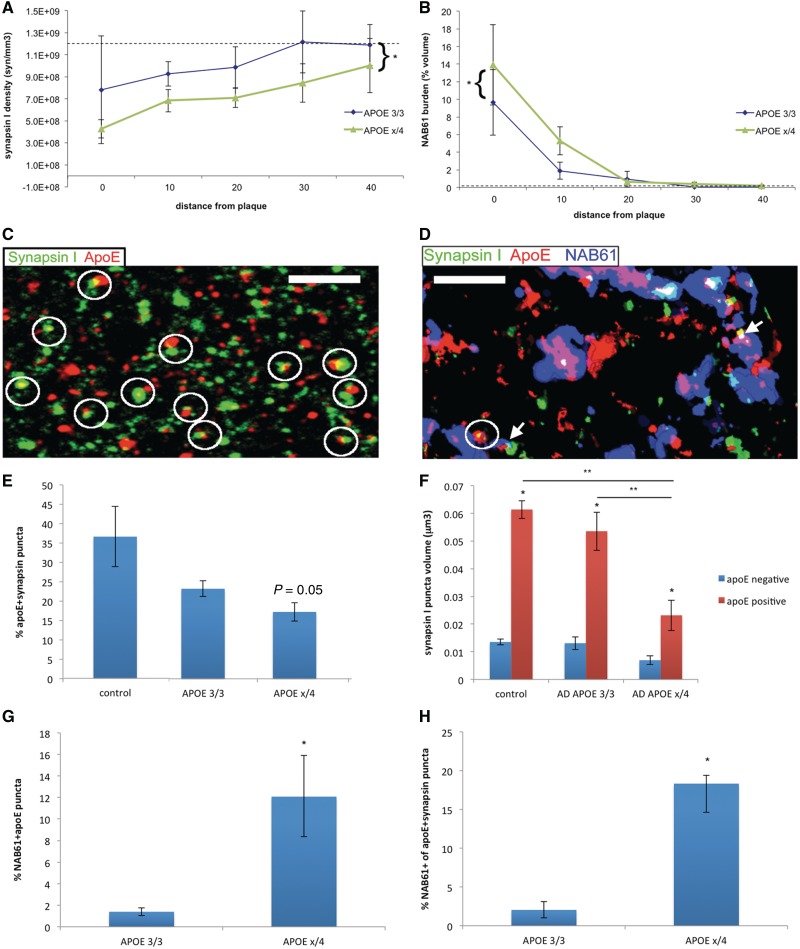
Figure 3.
ApoE4 increases accumulation of oligomeric amyloid-β at synaptic sites. Array tomograms of brains of Alzheimer’s disease cases reveal that synapse density near plaques (<50 µm away from nearest plaque) is lower in cases with one or two copies of ε4 (APOE ε3/4 or APOE ε4/4, combined referred to as APOE εx/4) compared with APOE ε3/3 cases (A). In this same region around plaques, oligomeric amyloid-β burden (represented by neuropil volume occupied by NAB61 staining) is higher in APOE εx/4 cases (B). Using array tomograms stained with apoE, NAB61 and synapsin I, we found that apoE is localized to a subset of synapses. A z-projection of four consecutive array tomogram sections stained with apoE and synapsin I in a control brain (C) shows the localization of apoE at presynaptic sites (circles). In Alzheimer’s disease brains near plaques (within 50 µm), we observe that apoE and oligomeric amyloid-β can be present in the same synapses (arrows show examples of synapses containing both apoE and oligomeric amyloid-β in a 3D reconstruction of 14 sections in D). There is a genotype-dependent effect in apoE association with synapses, with apoE3 being more likely to co-localize with synaptic elements than apoE4 (E, P = 0.05 Wilcoxon test). ApoE positive synapsin I puncta are significantly larger than apoE negative puncta in each group (F, *P < 0.05 Wilcoxon test apoE positive versus apoE negative synapsin volume). In APOE εx/4 cases, this apoE associated increase in synapse volume is not as pronounced as in control and APOE ε3/3 cases (post hoc Wilcoxon each pair **P < 0.05 for control and APOE ε3/3 versus APOE εx/4 apoE positive synapsin volume). ApoE co-localizes with oligomeric amyloid-β in an isoform dependent fashion, with apoE4 being more likely to co-localize with oligomeric amyloid-β than apoE3 (G). Furthermore, apoE4-positive synaptic elements are more likely to be positive for oligomeric amyloid-β than apoE3-positive synapses (*P < 0.05 APOE3/3 vs APOEx/4 Wilcoxon test) (H). Scale bars = 3 µm.
Next, we carried out high-resolution single synapse analysis on >30 000 synapses in Alzheimer’s disease and control brains to determine whether apoE localizes to synapses or influences oligomeric amyloid-β synaptic localization. All synapses analysed in each case were characterized as NAB61-positive or -negative and apoE-positive or -negative and the numbers of these subclasses were divided by the total number of synapsin puncta analysed to determine the percentages for each case. Medians of cases in each genotype were compared using non-parametric tests. Simultaneous immunostaining of array tomography preparations for apoE, oligomeric amyloid-β and presynaptic elements demonstrated that apoE is a synaptic protein that co-localizes with synaptic elements to a marked extent (∼18–36% of synapses contain apoE) in both control and Alzheimer’s disease brains (Fig. 3E). In patients with Alzheimer’s disease with one or two copies of E4, there is a trend towards fewer apoE-positive synapsin puncta (P = 0.05 compared with controls, Wilcoxon all pairs). ApoE-positive synapses, in contrast to the effect we see with the presence of oligomeric amyloid-β, are larger in both control and Alzheimer’s disease brains (Fig. 3F). Synapses in all cases were larger in the presence of apoE, but in the APOE εx/4 cases, the apoE positive synapses are not as large as those in control or ε3/3 cases (Fig. 3F, Kruskal–Wallis test, presence of ε4 P = 0.007, post hoc Wilcoxon each pair εx/4 apoE positive synapsin puncta significantly smaller than in APOE ε3/3, and than in control cases, P < 0.05).
Along with exacerbated synaptic loss around plaques, APOE εx/4 cases have significantly higher co-localization of oligomeric amyloid-β and apoE than APOE ε3/3 cases (Wilcoxon test, P = 0.04, Fig. 3G). Examined from the perspective of apoE positive synapses, there is an even more dramatic effect of APOE genotype. We found that 18.3% of apoE-positive synapses are also positive for oligomeric amyloid-β in Alzheimer’s disease APOE εx/4 cases compared with 2.0% in APOE ε3/3 subjects (Fig. 3H, Wilcoxon test, P < 0.05). The observed co-localization of oligomeric amyloid-β and synapses is many fold higher when apoE is present at the synapse than when it is absent, especially for APOE ε4 cases. For example, the rate of 18.3% of apoE4 positive synapses also positive for oligomeric amyloid-β is >5-fold higher than the co-localization rate of 4.5% (range 0–6.3%) of synapses not positive for apoE which contained oligomeric amyloid-β in the same brains, suggesting that apoE (especially apoE4) enhances accumulation of oligomeric amyloid-β at synapses.
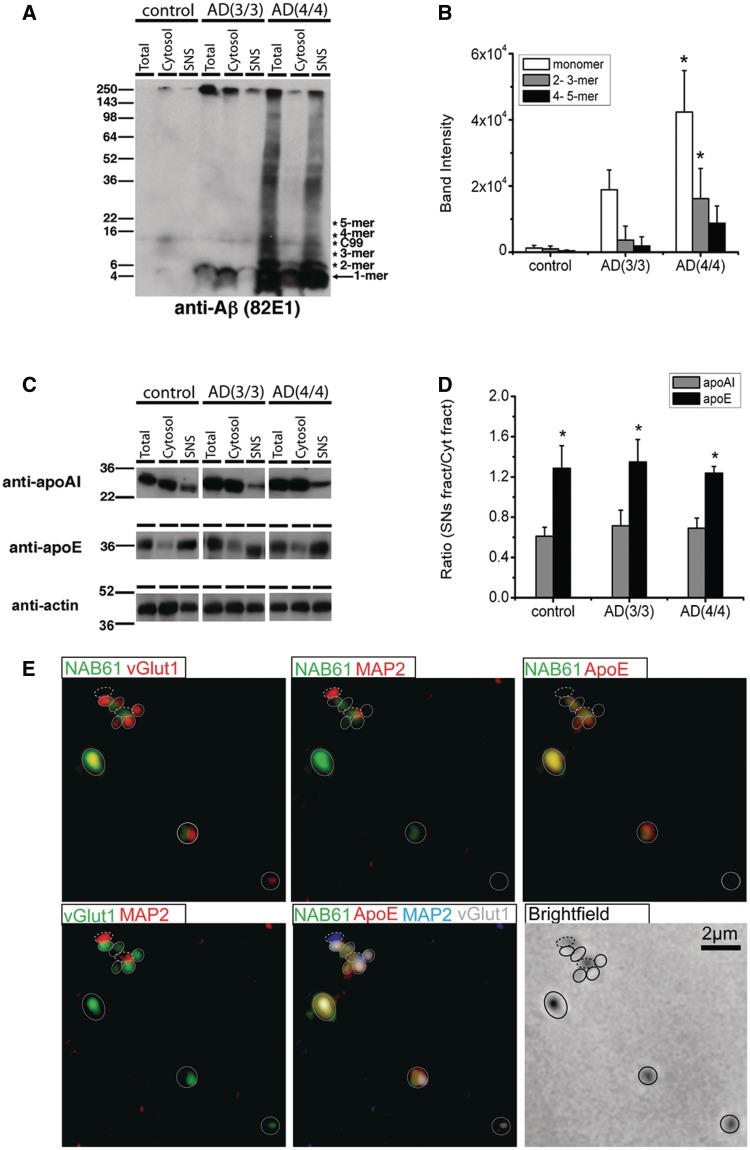
To confirm with a different method that oligomeric amyloid-β is present at synapses and to further characterize the species of oligomeric amyloid-β present at synapses and their relationship to different APOE isoforms, we carried out a series of biochemical fractionation experiments using human Alzheimer’s disease and control brains. For these experiments, Alzheimer’s patients with APOE ε3/3 (n = 6) and APOE ε4/4 (n = 6) genotypes were carefully selected to have equal plaque burden in frontal cortex as assessed by stereological histopathological analysis (mean 3/3 burden 3.0 ± 1.3%, mean 4/4 burden 3.8 ± 2.7%, P > 0.05 t-test). Six control subjects with different APOE genotypes were also selected (Supplementary Table 2). We prepared synaptoneurosome and cytosolic fractions from frontal cortex brain homogenates of these subjects and carried out SDS-PAGE followed by western blots. We verified that the synaptoneurosome preparations were enriched in PSD95 and synaptophysin, while actin was unchanged (Supplementary Fig. 2). SDS-PAGE and western blotting with the high affinity amyloid-β antibody 82E1 reveals that SDS-stable oligomers of amyloid-β are robustly enriched in synaptoneurosome fractions in patients with Alzheimer’s disease with APOE ε4/4 compared with patients with Alzheimer’s disease with APOE ε3/3 genotype and controls (Fig. 4A). The levels of synaptic amyloid-β monomers and SDS-stable dimers and trimers of amyloid-β were 2- and 4-fold higher, respectively, in APOE ε4/4 individuals with Alzheimer’s disease compared with patients with Alzheimer’s disease with APOE ε3/3 genotype (Fig. 4B). SDS-PAGE of the same samples and western blotting for apoE and apoAI reveals that, regardless of isoform, apoE is enriched at synapses whereas apoAI is not (Fig. 4C and D). Several apoE fragments were observed in synaptoneurosome fractions from APOE ε4/4 Alzheimer’s disease patients’ brains but not APOE ε3/3 Alzheimer’s disease brains (Supplementary Fig. 3). ApoE is enriched at synapses to the same extent in controls and subjects with Alzheimer’s disease, suggesting that the presence of apoE at synapses is not pathological.
Figure 4.
Biochemical fractionation confirms synaptic localization of oligomeric amyloid-β and ApoE. Biochemical fractionation experiments of brain homogenates from patients with Alzheimer’s disease with APOE ε3/3 (n = 6) or APOE ε4/4 (n = 6) matched for plaque burden, and controls (n = 6) show that APOE ε4 is associated with increased amyloid-β oligomers and monomers in synaptoneurosomes (A and B). Since subjects with Alzheimer’s disease were carefully matched to have similar plaque burdens through stereologic histopathological analysis, these results were not due to higher amyloid-β burden in APOE ε4/4 brains. ApoE is significantly enriched in synaptoneuromes in both Alzheimer’s disease and control brains, whereas apoAI is not (actin served as loading control) (C). There is no isoform specific difference in the level of enrichment of apoE in synaptoneurosomes (D). To confirm that apoE and oligomeric amyloid-β are present at a subset of both pre- and postsynaptic elements as observed with array tomography, synaptoneurosome preparations were spread thinly on a slide and immunostained for oligomeric amyloid-β (NAB61), apoE, presynaptic marker vGlut1 and postsynaptic dendritic marker MAP2 (E). These preparations (pseudocoloured in the two channel images so that co-localized puncta appear yellow) confirm the presence of both oligomeric amyloid-β and apoE at a subset of isolated pre- (closed circles) and postsynaptic elements (dashed circles). For (B) *P < 0.05 one-way ANOVA, Bonferroni test; for (D) *P < 0.05 compared with apoAI in each group. AD = Alzheimer’s disease.
These biochemical results independently confirm that apoE is associated with synapses in human brains. Further, they show using 82E1, a different antibody from the conformation-specific NAB61 used in array tomography, that SDS-stable dimers and trimers of amyloid-β, which have been shown to be synaptotoxic (Shankar et al., 2008), are enriched at synapses in APOE ε4/4 patients with Alzheimer’s disease. Synaptoneurosomes contain both pre- and postsynaptic elements (Fig. 4E) thus these biochemical data do not differentiate between pre- and postsynaptic localization of apoE and amyloid-β. To further confirm the array data showing both pre- and postsynaptic localization of these proteins, we developed a new technique combining biochemical isolation of synaptoneurosomes with immunofluorescence. Synaptoneurosomes isolated from patients with Alzheimer’s disease and controls as used for the biochemical studies were spread in a thin layer on a slide, stained with synaptic markers, NAB61 and apoE antibodies, and imaged using immunofluorescence and brightfield microscopy (Fig. 4E). We can clearly see isolated presynaptic and postsynaptic puncta as well as ‘snowman’ structures with the pre- and postsynaptic elements still attached. Immunofluorescence confirms the presence of NAB61 positive amyloid-β and ApoE at a subset of both pre- and postsynaptic elements. Together with the array tomography data, these results demonstrate that apoE and amyloid-β oligomers occur at synapses in Alzheimer’s disease, and that this occurs most often with apoE4.
Apolipoprotein E4 enhances localization of oligomeric amyloid-β with synaptic elements in vitro
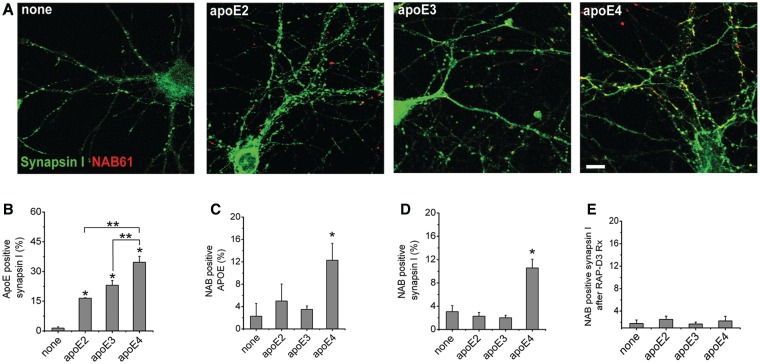
The results from array tomography and biochemical evaluation of human Alzheimer’s disease brains suggest that apoE4 may be enhancing accumulation of oligomeric amyloid-β at synapses by either targeting oligomeric amyloid-β to the synapse or less efficiently clearing it from the synapse. We examined these questions in a mouse primary neuronal culture system to distinguish between these possible mechanisms of apoE4 mediated enhanced synaptic localization of oligomeric amyloid-β. Cultured neurons were exposed to oligomeric amyloid-β containing media as previously described (Wu et al., 2010) and lipidated apoE particles (Morikawa et al., 2005) for 48 h, and the level of co-localization of apoE, oligomeric amyloid-β and synaptic elements determined. We found that while all isoforms of apoE co-localize with synaptic elements (P < 0.01 for each condition compared with no apoE treatment), only apoE4 enhances localization of oligomeric amyloid-β to synapses (Fig. 5A and B). This effect is robust: oligomeric amyloid-β associates with 10.6 ± 1.5% (P < 0.05) of synaptic elements in vitro in the presence of apoE4 compared with 2.2 ± 0.6% [not significant (NS)], 2.0 ± 0.4% (NS), and 3.0 ± 0.7% for apoE2, apoE3 and no apoE treatment, respectively (Fig. 5D). Further, we found that 12.3 ± 6.0% of apoE4 puncta were positive for oligomeric amyloid-β compared with 5.0 ± 3.0% and 3.5 ± 0.6% for apoE2 and apoE3, respectively (Fig. 5C). This experiment, using exogenously applied oligomeric amyloid-β and lipidated apoE, supports the hypothesis that apoE4 affects localization of oligomeric amyloid-β to synapses rather than having a primary effect on clearance of oligomeric amyloid-β from the synapse.
Figure 5.
ApoE4 enhances colocalization of oligomeric amyloid-β with synaptic elements in vitro. For in vitro experiments, cultured mouse neurons were treated with oligomeric amyloid-β-containing media and lipidated apoE particles for 48 h, fixed, permeabilized and immunostained to determine the level of colocalization between oligomeric amyloid-β, apoE and presynaptic elements. ApoE4 treated cultured neurons showed enhanced localization of oligomeric amyloid-β at synaptic sites (A). While all isoforms of apoE colocalized with synaptic elements to a marked extent (B), only apoE4 colocalized significantly with oligomeric amyloid-β in vitro (C). Further, apoE4, but not apoE2 or apoE3, enhances colocalization of oligomeric amyloid-β with synaptic elements (D) (n = 4 replicates per group, 4767 synapses). Treating cultured neurons with RAP-D3 to block low-density lipoprotein-receptor family members for 24 h under the same conditions as above abolished the apoE4 isoform specific accumulation of oligomeric amyloid-β at synaptic sites (E). *P < 0.01 Mann–Whitney test compared with no treatment, **P < 0.05 compared with indicated treatment group. Data shown as mean ± SEM. Scale bar = 5 µm.
To determine whether functional apoE receptors are necessary for apoE4-dependent accumulation of oligomeric amyloid-β at synaptic sites, we treated cultured neurons for 24 h with lipidated apoE particles and oligomeric amyloid-β containing media both in the presence or absence of an apoE-receptor antagonist, receptor associated protein domain 3 (RAP-D3) (Fisher et al., 2006; Blacklow, 2007). RAP-D3 treatment abolished the apoE4-dependent increase of oligomeric amyloid-β localization to synaptic elements (Fig. 5E), suggesting that apoE4 targets oligomeric amyloid-β to synaptic sites via a mechanism that requires functional synaptic apoE receptors.
Discussion
It is well established that the APOE ε4 allele greatly increases the risk for developing Alzheimer’s disease (Strittmatter et al., 1993). Similarly, it is known that synapse loss is a strong pathological correlate of cognitive decline in Alzheimer’s disease (DeKosky and Scheff, 1990). Here we provide evidence from human Alzheimer’s disease brain linking APOE ε4, synapse loss and oligomeric amyloid-β.
Several lines of evidence from animal studies implicate soluble amyloid-β oligomers as the molecular trigger of synapse collapse (Walsh et al., 2002, 2005; Shankar et al., 2007, 2008; Welsby et al., 2007; Li et al., 2009). We tested the hypothesis that this is the case in human Alzheimer’s disease as well. We first asked whether oligomeric amyloid-β is related to synaptic impairment in human Alzheimer’s disease and demonstrate that: (i) synapse density decreases progressively approaching plaques in the human brain from normal levels at distances >50 µm away from the nearest plaque to ∼35% of normal level at the edge of plaques; (ii) oligomeric amyloid-β is associated with synapses around plaques and in the neuropil; and (iii) the burden of oligomeric amyloid-β correlates with synapse loss.
We postulated that oligomeric amyloid-β surrounding senile plaques and extending into the neuropil interact with a subset of synapses to instigate synapse collapse in human Alzheimer’s disease patient brains. Indeed, we found that ∼15% of synapses are positive for oligomeric amyloid-β at distances <20 µm away from plaques. Further, the burden of oligomeric amyloid-β correlates with the extent of synapse loss, linking oligomeric amyloid-β interaction with synaptic elements in the mechanism underlying synapse loss. The presence of oligomeric amyloid-β at synapses was confirmed in three ways: first on array tomography with a conformation specific antibody (NAB61), which preferentially recognizes oligomeric forms of amyloid-β; second with an independent, well characterized antibody that recognizes all conformations of amyloid-β (R1282) on array tomography and third by synaptoneurosome preparations showing with another independent antibody (82E1) that SDS-stable dimers and trimers as well as monomeric species (which could be true monomers in brain or broken down from non-SDS-stable oligomers) of amyloid-β are present in synapses.
We also found that synaptic elements that are positive for NAB61 immunostaining are significantly smaller than those not in contact with NAB61-positve puncta. No technique is available to biochemically identify which amyloid-β species is present at these small synapses, but previous preclinical studies have demonstrated that oligomeric, but not monomeric, amyloid-β impacts neuronal physiology and dendritic spine stability (Cleary et al., 2005; Spires et al., 2005; Shankar et al., 2007; Li et al., 2009). Thus, while we cannot rule out the presence of other non-NAB61 immunoreactive species that could, in principle, co-localize with NAB61 at a synapse and act as a potent synaptotoxin, the most parsimonious explanation is that NAB61 immunopositive oligomeric amyloid-β species are directly synaptotoxic in human brain. Our data therefore support the hypothesis that oligomeric amyloid-β is directly toxic to synapses in the Alzheimer’s disease brain and that amyloid-β oligomers induce changes in synapses that lead to synapse shrinkage and loss. Further, we find that oligomeric amyloid-β-positive synaptic elements in control brains are also smaller than those not in contact with amyloid-β oligomers, further reinforcing the idea that oligomeric amyloid-β has potent synaptic effects.
We next examined whether the APOE ε4 allele influenced oligomeric amyloid-β levels in the brains of Alzheimer’s disease patients, and if so, if it increased synapse loss. Our results using array tomography show clearly that APOE ε4 is associated with higher oligomeric amyloid-β levels and marked loss of synapses. We found using single synapse analysis of >30 000 synapses, that apoE co-localized with oligomeric amyloid-β over three times as often in patients carrying the APOE ε4 allele than in those with only APOE ε3 alleles and that apoE-positive synaptic elements are 5-fold more likely to co-localize with oligomeric amyloid-β in the brains of APOE ε4 patients than synaptic elements positive for apoE in APOE ε3 brains. Thus we postulate that the apoE4 isoform specifically targets oligomeric amyloid-β to synapses more efficiently than apoE3. We cannot rule out a weaker effect of apoE4 on clearance of oligomeric amyloid-β from synaptic sites from post-mortem studies, and recognize that apoE may have pleiotropic effects on both delivery of oligomeric amyloid-β to synapses and on its potential uptake or clearance either at the synapse or by competing mechanisms (Castellano et al., 2011). We confirmed the apoE mediated targeting of oligomeric amyloid-β to synapses by apoE4 using cultured neurons, where we observed that apoE4, but not apoE2 or apoE3, enhances co-localization of oligomeric amyloid-β with synaptic elements via a mechanism involving apoE receptors. ApoE protein was confirmed to be present at synapses in synaptoneurosome preparations, which also showed that APOE ε4/4 genotype is associated with increased synaptotoxic dimers and trimers of amyloid-β at synapses. Taken together, our results strongly suggest that the apoE4 isoform enhances localization of synaptotoxic oligomeric amyloid-β species to synapses.
Our data predict that any situation in which increased amounts of apoE is synthesized (particularly apoE4) would lead to increased oligomeric amyloid-β-mediated synaptotoxicity. This model is in contrast with the recent report of beneficial effects of retinoid X receptor agonist treatment of Alzheimer’s disease model mice, which caused increased apoE levels (Cramer et al., 2012), but it is in agreement with the many studies in mice showing that reducing apoE levels reduces amyloid pathology and improves cognition in Alzheimer’s disease models (Holtzman et al., 2000; Irizarry et al., 2000; Kim et al., 2011). We hypothesize that the well-established haplotype effects of APOE (Chartier-Harlin et al., 1994; Lambert et al., 2002) and the worse cognitive outcomes seen for APOE ε4 patients who suffer brain injury (Nicoll et al., 1996; Friedman et al., 1999; Lynch et al., 2002; Teasdale et al., 2005) might reflect the effects of increased amounts of apoE4 and apoE4/oligomeric amyloid-β on synaptic stability.
Our results do not preclude other potential mechanisms whereby apoE4 might impact Alzheimer’s disease biology. A role for apoE in amyloid-β clearance or stabilization may mediate the increase in amyloid-β burden observed in APOE ε4 cases (Kim et al., 2009; Reiman et al., 2009; Morris et al., 2010; Castellano et al., 2011). The interactions between apoE and its receptors, including the reelin receptors VLDL-r and apoER2, may influence synaptic stability (Hoe and Rebeck, 2008; Herz, 2009), and differential effects of apoE3 and apoE4 on neuronal phenotypes such as neurite outgrowth have been established (Nathan et al., 1994; Bellosta et al., 1995; Sun et al., 1998). ApoE receptors also have a complex relationship with amyloid precursor protein, leading to bilateral interactions altering metabolism of both (Kounnas et al., 1995; Liu et al., 2007). Nonetheless, our data, derived from direct evaluation of human Alzheimer’s disease patients, suggest a simple mechanism in which apoE4 protein has an over 5-fold enhanced localization of oligomeric amyloid-β to the synapse compared with apoE3, thus directly accelerating its synaptotoxic effects.
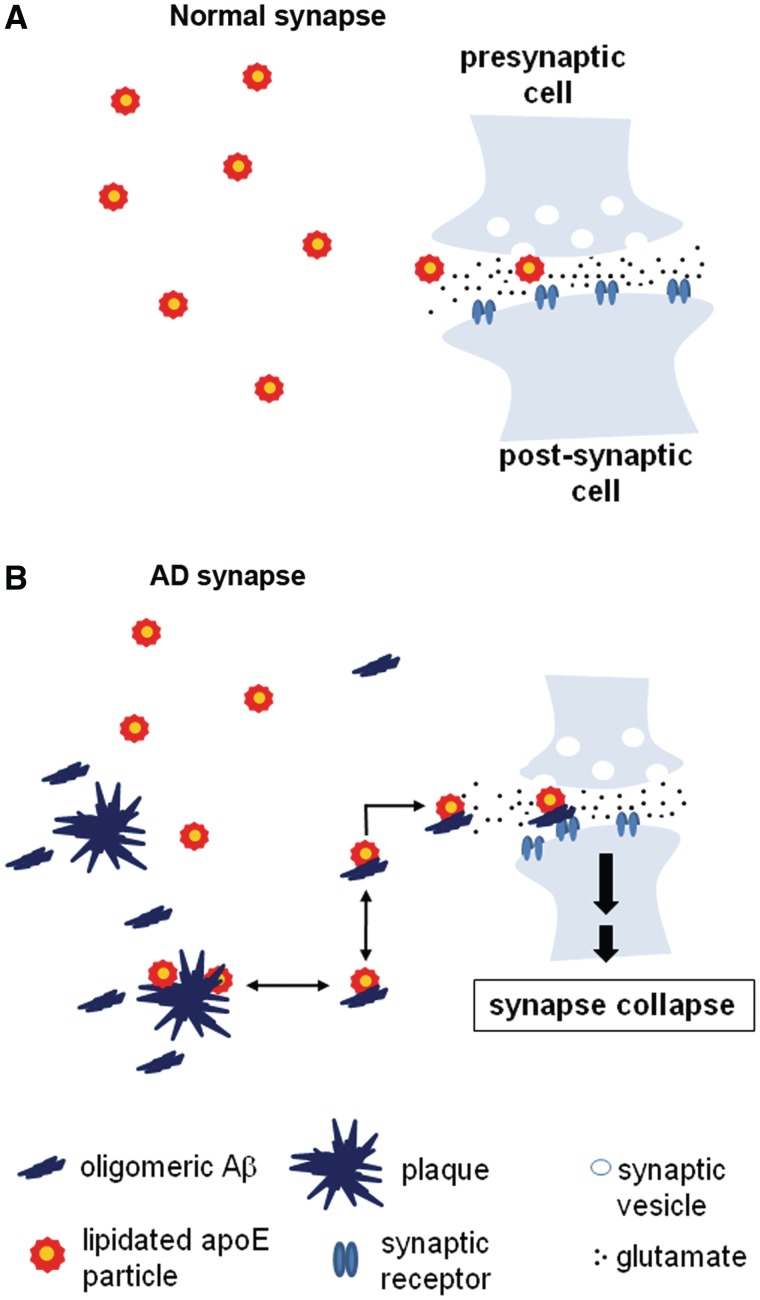
In summary, we examined the relationship of oligomeric amyloid-β to synapse loss in human Alzheimer’s disease along with the effect of APOE genotype, using a new synaptic analysis tool, array tomography. We demonstrate that apoE4 increases levels of oligomeric amyloid-β and targets it to the synapse where it contributes to synapse shrinkage and loss in Alzheimer’s disease brains. The apoE effect on synapse–oligomeric amyloid-β interaction occurs in an isoform-specific fashion (E4>E3), providing a direct and simple mechanistic link between the strongest genetic risk factor for sporadic Alzheimer’s disease (APOE ε4 genotype), the occurrence of a specific form of soluble, toxic amyloid-β (oligomeric amyloid-β) at synapses and the strongest correlate of cognitive impairment in Alzheimer’s disease, synapse loss. Together, these findings support the hypothesis that apoE4 facilitates association of oligomeric amyloid-β with synapses where it is directly toxic (Fig. 6). These data also strongly support the idea that oligomeric amyloid-β plays an important role in synaptic damage not only in animal models of amyloid-β toxicity, but in human Alzheimer’s disease as well. Since synapses can reform after damage, blockade of the oligomeric amyloid-β-apoE interaction could be a therapeutic approach to preventing amyloid-β mediated synapse loss in Alzheimer’s disease.
Figure 6.
Model of synaptic effects of oligomeric amyloid-β and apoE. (A) Normal synapses receive lipid support from lipidated apoE particles, which interact with synaptic apoE receptors and deliver lipids to neuronal synapses. (B) In Alzheimer’s disease (AD), however, these apoE particles, particularly lipidated apoE4 particles, tend to stabilize synaptotoxic oligomeric amyloid-β species and inadvertently enhance their accumulation at synapses, leading to synapse shrinkage and loss.
Funding
NIH K99 (AG033670-01A1), Alzheimer’s disease Drug Discovery Foundation/Association for Frontotemporal Dementias, NIH grants P50 AG005134, AG12406, AG08487, Harvard Biophysics and Medical Scientist Training Programs, NIH T32 GM07753, Paul and Daisy Soros Foundation, Ellison/American Federation for Aging Research Postdoctoral Fellowship (2009A059868). American Health Assistance Foundation Alzheimer’s Disease Fellowship.
Supplementary material
Supplementary material is available at Brain online.
Acknowledgements
Authors thank Drs Stephen Smith and Kristina Micheva for advice on array tomography. Authors also thank Dr Stephen Blacklow for providing RAP-D3. They also thank Drs Kishore Kuchibhotla and Scott Raymond for MATLAB programming and expertise.
Glossary
Abbreviations
- APOE
apolipoprotein E
References
- Bellosta S, Nathan BP, Orth M, Dong LM, Mahley RW, Pitas RE. Stable expression and secretion of apolipoproteins E3 and E4 in mouse neuroblastoma cells produces differential effects on neurite outgrowth. J Biol Chem. 1995;270:27063–71. doi: 10.1074/jbc.270.45.27063. [DOI] [PubMed] [Google Scholar]
- Blacklow SC. Versatility in ligand recognition by LDL receptor family proteins: advances and frontiers. Curr Opin Struct Biol. 2007;17:419–26. doi: 10.1016/j.sbi.2007.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castellano JM, Kim J, Stewart FR, Jiang H, DeMattos RB, Patterson BW, et al. Human apoE isoforms differentially regulate brain amyloid-beta peptide clearance. Sci Transl Med. 2011;3:89ra57. doi: 10.1126/scitranslmed.3002156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chartier-Harlin MC, Parfitt M, Legrain S, Perez-Tur J, Brousseau T, Evans A, et al. Apolipoprotein E, epsilon 4 allele as a major risk factor for sporadic early and late-onset forms of Alzheimer's disease: analysis of the 19q13.2 chromosomal region. Hum Mol Genet. 1994;3:569–74. doi: 10.1093/hmg/3.4.569. [DOI] [PubMed] [Google Scholar]
- Cleary JP, Walsh DM, Hofmeister JJ, Shankar GM, Kuskowski MA, Selkoe DJ, et al. Natural oligomers of the amyloid-beta protein specifically disrupt cognitive function. Nat Neurosci. 2005;8:79–84. doi: 10.1038/nn1372. [DOI] [PubMed] [Google Scholar]
- Corder EH, Saunders AM, Strittmatter WJ, Schmechel DE, Gaskell PC, Small GW, et al. Gene dose of apolipoprotein E type 4 allele and the risk of Alzheimer’s disease in late onset families. Science. 1993;261:921–3. doi: 10.1126/science.8346443. [DOI] [PubMed] [Google Scholar]
- Cramer PE, Cirrito JR, Wesson DW, Lee CY, Karlo JC, Zinn AE, et al. ApoE-directed therapeutics rapidly clear beta-amyloid and reverse deficits in AD mouse models. Science. 2012;335:1503–6. doi: 10.1126/science.1217697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeKosky ST, Scheff SW. Synapse loss in frontal cortex biopsies in Alzheimer’s disease: correlation with cognitive severity. Ann Neurol. 1990;27:457–64. doi: 10.1002/ana.410270502. [DOI] [PubMed] [Google Scholar]
- Fisher C, Beglova N, Blacklow SC. Structure of an LDLR-RAP complex reveals a general mode for ligand recognition by lipoprotein receptors. Mol Cell. 2006;22:277–83. doi: 10.1016/j.molcel.2006.02.021. [DOI] [PubMed] [Google Scholar]
- Friedman G, Froom P, Sazbon L, Grinblatt I, Shochina M, Tsenter J, et al. Apolipoprotein E-epsilon4 genotype predicts a poor outcome in survivors of traumatic brain injury. Neurology. 1999;52:244–8. doi: 10.1212/wnl.52.2.244. [DOI] [PubMed] [Google Scholar]
- Gaspar RC, Villarreal SA, Bowles N, Hepler RW, Joyce JG, Shughrue PJ. Oligomers of beta-amyloid are sequestered into and seed new plaques in the brains of an AD mouse model. Exp Neurol. 2010;223:394–400. doi: 10.1016/j.expneurol.2009.09.001. [DOI] [PubMed] [Google Scholar]
- Gomez-Isla T, West HL, Rebeck GW, Harr SD, Growdon JH, Locascio JJ, et al. Clinical and pathological correlates of apolipoprotein E epsilon 4 in Alzheimer’s disease. Ann Neurol. 1996;39:62–70. doi: 10.1002/ana.410390110. [DOI] [PubMed] [Google Scholar]
- Herz J. Apolipoprotein E receptors in the nervous system. Curr Opin Lipidol. 2009;20:190–6. doi: 10.1097/MOL.0b013e32832d3a10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoe HS, Rebeck GW. Functional interactions of APP with the apoE receptor family. J Neurochem. 2008;106:2263–71. doi: 10.1111/j.1471-4159.2008.05517.x. [DOI] [PubMed] [Google Scholar]
- Hollingsworth EB, McNeal ET, Burton JL, Williams RJ, Daly JW, Creveling CR. Biochemical characterization of a filtered synaptoneurosome preparation from guinea pig cerebral cortex: cyclic adenosine 3′:5′-monophosphate-generating systems, receptors, and enzymes. J Neurosci. 1985;5:2240–53. doi: 10.1523/JNEUROSCI.05-08-02240.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holtzman DM, Fagan AM, Mackey B, Tenkova T, Sartorius L, Paul SM, et al. Apolipoprotein E facilitates neuritic and cerebrovascular plaque formation in an Alzheimer’s disease model. Ann Neurol. 2000;47:739–47. [PubMed] [Google Scholar]
- Irizarry MC, Rebeck GW, Cheung B, Bales K, Paul SM, Holzman D, et al. Modulation of A beta deposition in APP transgenic mice by an apolipoprotein E null background. Ann NY Acad Sci. 2000;920:171–8. doi: 10.1111/j.1749-6632.2000.tb06919.x. [DOI] [PubMed] [Google Scholar]
- Jones PB, Adams KW, Rozkalne A, Spires-Jones TL, Hshieh TT, Hashimoto T, et al. Apolipoprotein E: isoform specific differences in tertiary structure and interaction with amyloid-beta in human Alzheimer brain. PLoS One. 2010;6:e14586. doi: 10.1371/journal.pone.0014586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J, Basak JM, Holtzman DM. The role of apolipoprotein E in Alzheimer’s disease. Neuron. 2009;63:287–303. doi: 10.1016/j.neuron.2009.06.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J, Jiang H, Park S, Eltorai AE, Stewart FR, Yoon H, et al. Haploinsufficiency of human APOE reduces amyloid deposition in a mouse model of amyloid-beta amyloidosis. J Neurosci. 2011;31:18007–12. doi: 10.1523/JNEUROSCI.3773-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koffie RM, Hyman BT, Spires-Jones TL. Alzheimer’s disease: synapses gone cold. Mol Neurodegener. 2011;6:63. doi: 10.1186/1750-1326-6-63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koffie RM, Meyer-Luehmann M, Hashimoto T, Adams KW, Mielke ML, Garcia-Alloza M, et al. Oligomeric amyloid beta associates with postsynaptic densities and correlates with excitatory synapse loss near senile plaques. Proc Natl Acad Sci USA. 2009;106:4012–17. doi: 10.1073/pnas.0811698106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kounnas MZ, Moir RD, Rebeck GW, Bush AI, Argraves WS, Tanzi RE, et al. LDL receptor-related protein, a multifunctional ApoE receptor, binds secreted beta-amyloid precursor protein and mediates its degradation. Cell. 1995;82:331–40. doi: 10.1016/0092-8674(95)90320-8. [DOI] [PubMed] [Google Scholar]
- Lacor PN, Buniel MC, Furlow PW, Clemente AS, Velasco PT, Wood M, et al. Abeta oligomer-induced aberrations in synapse composition, shape, and density provide a molecular basis for loss of connectivity in Alzheimer’s disease. J Neurosci. 2007;27:796–807. doi: 10.1523/JNEUROSCI.3501-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambert JC, Araria-Goumidi L, Myllykangas L, Ellis C, Wang JC, Bullido MJ, et al. Contribution of APOE promoter polymorphisms to Alzheimer’s disease risk. Neurology. 2002;59:59–66. doi: 10.1212/wnl.59.1.59. [DOI] [PubMed] [Google Scholar]
- Lee EB, Leng LZ, Zhang B, Kwong L, Trojanowski JQ, Abel T, et al. Targeting amyloid-beta peptide (Abeta) oligomers by passive immunization with a conformation-selective monoclonal antibody improves learning and memory in Abeta precursor protein (APP) transgenic mice. J Biol Chem. 2006;281:4292–9. doi: 10.1074/jbc.M511018200. [DOI] [PubMed] [Google Scholar]
- Li S, Hong S, Shepardson NE, Walsh DM, Shankar GM, Selkoe D. Soluble oligomers of amyloid Beta protein facilitate hippocampal long-term depression by disrupting neuronal glutamate uptake. Neuron. 2009;62:788–801. doi: 10.1016/j.neuron.2009.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Q, Zerbinatti CV, Zhang J, Hoe HS, Wang B, Cole SL, et al. Amyloid precursor protein regulates brain apolipoprotein E and cholesterol metabolism through lipoprotein receptor LRP1. Neuron. 2007;56:66–78. doi: 10.1016/j.neuron.2007.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynch JR, Pineda JA, Morgan D, Zhang L, Warner DS, Benveniste H, et al. Apolipoprotein E affects the central nervous system response to injury and the development of cerebral edema. Ann Neurol. 2002;51:113–17. doi: 10.1002/ana.10098. [DOI] [PubMed] [Google Scholar]
- Mc Donald JM, Savva GM, Brayne C, Welzel AT, Forster G, Shankar GM, et al. The presence of sodium dodecyl sulphate-stable Abeta dimers is strongly associated with Alzheimer-type dementia. Brain. 2010;133(Pt 5):1328–41. doi: 10.1093/brain/awq065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLean CA, Cherny RA, Fraser FW, Fuller SJ, Smith MJ, Beyreuther K, et al. Soluble pool of Abeta amyloid as a determinant of severity of neurodegeneration in Alzheimer’s disease. Ann Neurol. 1999;46:860–6. doi: 10.1002/1531-8249(199912)46:6<860::aid-ana8>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- Micheva KD, Busse B, Weiler NC, O’Rourke N, Smith SJ. Single-synapse analysis of a diverse synapse population: proteomic imaging methods and markers. Neuron. 2010;68:639–53. doi: 10.1016/j.neuron.2010.09.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Micheva KD, Smith SJ. Array tomography: a new tool for imaging the molecular architecture and ultrastructure of neural circuits. Neuron. 2007;55:25–36. doi: 10.1016/j.neuron.2007.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morikawa M, Fryer JD, Sullivan PM, Christopher EA, Wahrle SE, DeMattos RB, et al. Production and characterization of astrocyte-derived human apolipoprotein E isoforms from immortalized astrocytes and their interactions with amyloid-beta. Neurobiol Dis. 2005;19:66–76. doi: 10.1016/j.nbd.2004.11.005. [DOI] [PubMed] [Google Scholar]
- Morris JC, Roe CM, Xiong C, Fagan AM, Goate AM, Holtzman DM, et al. APOE predicts amyloid-beta but not tau Alzheimer pathology in cognitively normal aging. Ann Neurol. 2010;67:122–31. doi: 10.1002/ana.21843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nathan BP, Bellosta S, Sanan DA, Weisgraber KH, Mahley RW, Pitas RE. Differential effects of apolipoproteins E3 and E4 on neuronal growth in vitro. Science. 1994;264:850–2. doi: 10.1126/science.8171342. [DOI] [PubMed] [Google Scholar]
- Nicoll JA, Roberts GW, Graham DI. Amyloid beta-protein, APOE genotype and head injury. Ann NY Acad Sci. 1996;777:271–5. doi: 10.1111/j.1749-6632.1996.tb34431.x. [DOI] [PubMed] [Google Scholar]
- Rebeck GW, Reiter JS, Strickland DK, Hyman BT. Apolipoprotein E in sporadic Alzheimer’s disease: allelic variation and receptor interactions. Neuron. 1993;11:575–80. doi: 10.1016/0896-6273(93)90070-8. [DOI] [PubMed] [Google Scholar]
- Reiman EM, Caselli RJ, Yun LS, Chen K, Bandy D, Minoshima S, et al. Preclinical evidence of Alzheimer’s disease in persons homozygous for the epsilon 4 allele for apolipoprotein E. N Engl J Med. 1996;334:752–8. doi: 10.1056/NEJM199603213341202. [DOI] [PubMed] [Google Scholar]
- Reiman EM, Chen K, Alexander GE, Caselli RJ, Bandy D, Osborne D, et al. Correlations between apolipoprotein E epsilon4 gene dose and brain-imaging measurements of regional hypometabolism. Proc Natl Acad Sci USA. 2005;102:8299–302. doi: 10.1073/pnas.0500579102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reiman EM, Chen K, Liu X, Bandy D, Yu M, Lee W, et al. Fibrillar amyloid-beta burden in cognitively normal people at 3 levels of genetic risk for Alzheimer’s disease. Proc Natl Acad Sci USA. 2009;106:6820–5. doi: 10.1073/pnas.0900345106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roses AD. Apolipoprotein E alleles as risk factors in Alzheimer’s disease. Annu Rev Med. 1996;47:387–400. doi: 10.1146/annurev.med.47.1.387. [DOI] [PubMed] [Google Scholar]
- Serrano-Pozo A, Mielke ML, Gomez-Isla T, Betensky RA, Growdon JH, Frosch MP, et al. Reactive glia not only associates with plaques but also parallels tangles in Alzheimer’s disease. Am J Pathol. 2010;179:1373–84. doi: 10.1016/j.ajpath.2011.05.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shankar GM, Bloodgood BL, Townsend M, Walsh DM, Selkoe DJ, Sabatini BL. Natural oligomers of the Alzheimer amyloid-beta protein induce reversible synapse loss by modulating an NMDA-type glutamate receptor-dependent signaling pathway. J Neurosci. 2007;27:2866–75. doi: 10.1523/JNEUROSCI.4970-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shankar GM, Li S, Mehta TH, Garcia-Munoz A, Shepardson NE, Smith I, et al. Amyloid-beta protein dimers isolated directly from Alzheimer’s brains impair synaptic plasticity and memory. Nat Med. 2008;14:837–42. doi: 10.1038/nm1782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spires TL, Meyer-Luehmann M, Stern EA, McLean PJ, Skoch J, Nguyen PT, et al. Dendritic spine abnormalities in amyloid precursor protein transgenic mice demonstrated by gene transfer and intravital multiphoton microscopy. J Neurosci. 2005;25:7278–87. doi: 10.1523/JNEUROSCI.1879-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strittmatter WJ, Saunders AM, Schmechel D, Pericak-Vance M, Enghild J, Salvesen GS, et al. Apolipoprotein E: high-avidity binding to beta-amyloid and increased frequency of type 4 allele in late-onset familial Alzheimer disease. Proc Natl Acad Sci USA. 1993;90:1977–81. doi: 10.1073/pnas.90.5.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun Y, Wu S, Bu G, Onifade MK, Patel SN, LaDu MJ, et al. Glial fibrillary acidic protein-apolipoprotein E (apoE) transgenic mice: astrocyte-specific expression and differing biological effects of astrocyte-secreted apoE3 and apoE4 lipoproteins. J Neurosci. 1998;18:3261–72. doi: 10.1523/JNEUROSCI.18-09-03261.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teasdale GM, Murray GD, Nicoll JA. The association between APOE epsilon4, age and outcome after head injury: a prospective cohort study. Brain. 2005;128(Pt 11):2556–61. doi: 10.1093/brain/awh595. [DOI] [PubMed] [Google Scholar]
- Thevenaz P, Ruttimann UE, Unser M. A pyramid approach to subpixel registration based on intensity. IEEE Trans Image Process. 1998;7:27–41. doi: 10.1109/83.650848. [DOI] [PubMed] [Google Scholar]
- Tomic JL, Pensalfini A, Head E, Glabe CG. Soluble fibrillar oligomer levels are elevated in Alzheimer’s disease brain and correlate with cognitive dysfunction. Neurobiol Dis. 2009;35:352–8. doi: 10.1016/j.nbd.2009.05.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walsh DM, Klyubin I, Fadeeva JV, Cullen WK, Anwyl R, Wolfe MS, et al. Naturally secreted oligomers of amyloid beta protein potently inhibit hippocampal long-term potentiation in vivo. Nature. 2002;416:535–9. doi: 10.1038/416535a. [DOI] [PubMed] [Google Scholar]
- Walsh DM, Townsend M, Podlisny MB, Shankar GM, Fadeeva JV, El Agnaf O, et al. Certain inhibitors of synthetic amyloid beta-peptide (Abeta) fibrillogenesis block oligomerization of natural Abeta and thereby rescue long-term potentiation. J Neurosci. 2005;25:2455–62. doi: 10.1523/JNEUROSCI.4391-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welsby PJ, Rowan MJ, Anwyl R. Beta-amyloid blocks high frequency stimulation induced LTP but not nicotine enhanced LTP. Neuropharmacology. 2007;53:188–95. doi: 10.1016/j.neuropharm.2007.05.013. [DOI] [PubMed] [Google Scholar]
- Wisniewski T, Lalowski M, Golabek A, Vogel T, Frangione B. Is Alzheimer’s disease an apolipoprotein E amyloidosis? Lancet. 1995;345:956–8. doi: 10.1016/s0140-6736(95)90701-7. [DOI] [PubMed] [Google Scholar]
- Wu HY, Hudry E, Hashimoto T, Kuchibhotla K, Rozkalne A, Fan Z, et al. Amyloid beta induces the morphological neurodegenerative triad of spine loss, dendritic simplification, and neuritic dystrophies through calcineurin activation. J Neurosci. 2010;30:2636–49. doi: 10.1523/JNEUROSCI.4456-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.