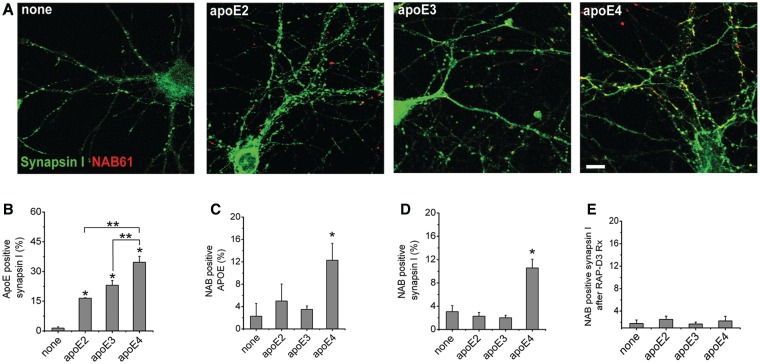
Figure 5.
ApoE4 enhances colocalization of oligomeric amyloid-β with synaptic elements in vitro. For in vitro experiments, cultured mouse neurons were treated with oligomeric amyloid-β-containing media and lipidated apoE particles for 48 h, fixed, permeabilized and immunostained to determine the level of colocalization between oligomeric amyloid-β, apoE and presynaptic elements. ApoE4 treated cultured neurons showed enhanced localization of oligomeric amyloid-β at synaptic sites (A). While all isoforms of apoE colocalized with synaptic elements to a marked extent (B), only apoE4 colocalized significantly with oligomeric amyloid-β in vitro (C). Further, apoE4, but not apoE2 or apoE3, enhances colocalization of oligomeric amyloid-β with synaptic elements (D) (n = 4 replicates per group, 4767 synapses). Treating cultured neurons with RAP-D3 to block low-density lipoprotein-receptor family members for 24 h under the same conditions as above abolished the apoE4 isoform specific accumulation of oligomeric amyloid-β at synaptic sites (E). *P < 0.01 Mann–Whitney test compared with no treatment, **P < 0.05 compared with indicated treatment group. Data shown as mean ± SEM. Scale bar = 5 µm.