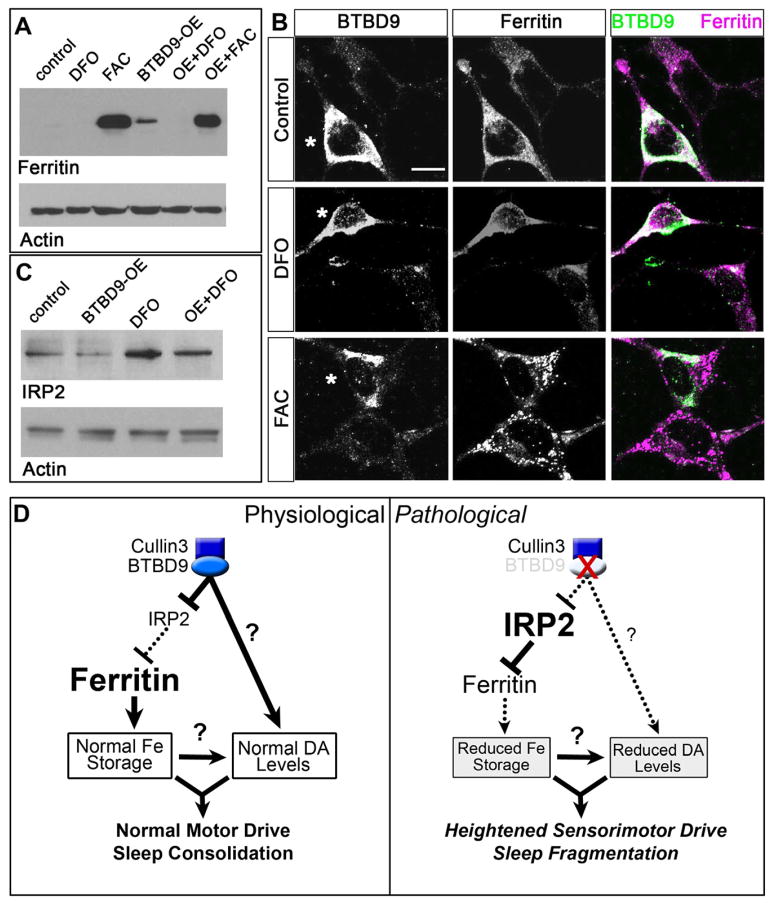
Figure 4. BTBD9 regulates iron metabolism by controlling ferritin expression via IRP2.
(A) Western blots of HEK cell protein lysates probed for ferritin light chain and actin (loading control). Ferritin levels are compared between untreated (control), deferoxamine mesylate (DFO) or ferric ammonium citrate (FAC) treated cells and BTBD9 transfected cells that are either untreated (BTBD9-OE) or treated with DFO or FAC. (B) BTBD9 and ferritin immunocytochemistry on HEK cells transfected with BTBD9 that are either untreated or treated with DFO or FAC. Asterisks mark BTBD9 transfected cells. Scale bar = 10 μm. (C) Western blot of HEK cell lysates probed for IRP2. DFO treated and untreated cells are compared with cells that are processed similarly but also over-express transgenic BTBD9. (D) Model of BTBD9 function under normal and pathological conditions. BTBD9 acts together with Cullin-3 to regulate IRP2 levels in the cell. This in turn controls ferritin expression and iron metabolism. Under normal conditions, BTBD9 inhibits IRP2 and promotes ferritin expression. BTBD9 also maintains dopamine biosynthesis either directly, or indirectly through iron, by currently unknown mechanisms. Together, this ensures normal motor drive and sleep consolidation. However, in RLS, loss of BTBD9 leads to increased IRP2, and reduced ferritin, thereby altering iron metabolism. It also negatively impacts dopamine synthesis leading to inappropriate motor activity and sleep fragmentation.