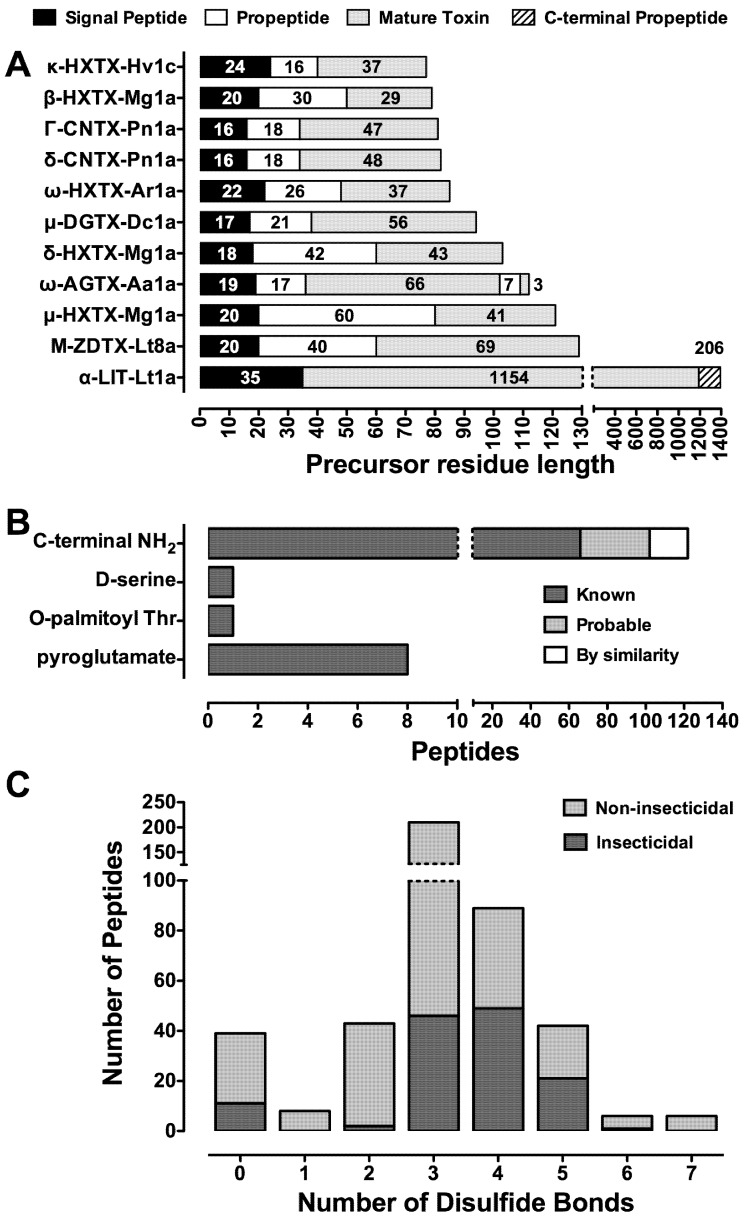
Figure 2.
Precursor architecture and posttranslational modifications in spider-venom peptides. (A) All insecticidal spider-venom toxins display a classical prepropeptide paradigm except LITs (e.g., α-LIT-Lt1a) from Latrodectus spp. ω-AGTX-Aa1a from Agelenopsis aperta is a heterodimer consisting of a 66-residue major chain that is linked via a disulfide bond to a 3-residue minor chain; (B) Known PTMs in spider-venom peptides (dark grey bars) as well as probable PTMs (light grey bars) and those predicted from sequence homology (white bars); (C) Distribution of the number of disulfide bonds found in insecticidal spider toxins (dark grey bars) and non-insecticidal peptides (light grey bars). Peptides with unknown disulfide connectivity are not shown. Note the discontinuous axes in all panels. Data were collated from the ArachnoServer 2.0 Spider Toxin Database (www.arachnoserver.org; [70], accessed on 20 January 2012).