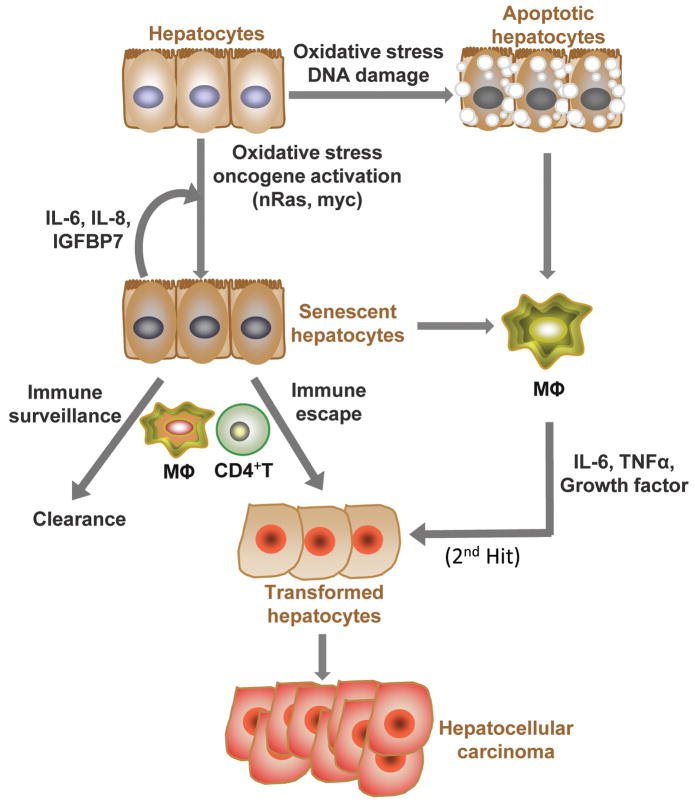
Figure 1. Avoiding hepatocellular oncogenesis through immune-mediated processes.
Failsafe mechanisms to counter malignant transformation of hepatocytes involve both cell intrinsic and extrinsic components. In response to cellular damage, cell intrinsic checkpoint pathways arrest cell growth (senescence, autophagy) and/or initiate programs that result in clearance (apoptosis). In the liver, these programs elicit specific extrinsic responses by local immune cells. During the progression to hepatocellular transformation, oncogene activation can also induce both apoptosis and senescence. Both processes result in release of cytokines and chemokines (SASP, senescence associated secretory phenotype and DAMPS, damage associated molecular patterns) into the microenvironment to recruit and instruct immune system cells to reinforce these processes. Innate immune components such as NK and KC stand watch for signs of oncogene-induced hepatocellular stress such as NKG2D and DAMPS (IL-1α, HMGb1). KC also serve as the primary source for IL-6 in the orchestration of compensatory proliferation required for both hepatic regeneration and tumor progression. CD4+ adaptive immune responses are mounted against oncogene induced senescent cells leading to clearance effected by macrophages. Oncogene-initiated cells may escape these mechanisms by acquiring additional or “2nd hits” in these pathways leading to transformation.