Abstract
Background
The mesocorticolimbic dopamine system mediates the reinforcing effects of salient stimuli, including drugs of abuse. Nondependent chronic alcohol consumption modifies this system, resulting in an increased number of spontaneously active dopamine neurons in the posterior ventral tegmental area (VTA) of alcohol-preferring (P) rats. Enhanced responses of postsynaptic glutamate receptors may contribute to the increase in active dopamine neurons. Thus, excitations of putative dopamine neurons to locally-applied NMDA (glutamate receptor subtype agonist) were evaluated.
Methods
P rats were assigned to alcohol naïve (water only) or alcohol drinking (continuous access to 15% alcohol and water for 8 consecutive weeks) groups. Responses of 23 putative dopamine neurons from naïve rats and 19 putative dopamine neurons from drinking rats were assessed in vivo using microiontophoretically-applied NMDA. Current-response curves for firing frequency and burst activity were constructed using nonlinear mixed effects models. Between-group comparisons were made for EC50 (effective current producing a half maximal excitatory response), Emax (maximal excitatory effect) and the CDB (the current at which depolarization block - marked decrease in neuronal activity - occurred).
Results
Drinking P rats steadily consumed alcohol over the eight-week protocol and did not exhibit signs of dependence or withdrawal. Putative dopamine neurons from drinking rats exhibited resistance to depolarization block (higher CDB values) and required larger doses of NMDA to elicit moderate excitatory responses (higher EC50 values), consistent with decreased receptor affinity. Maximal excitatory responses (Emax) did not differ between the groups, consistent with no change in receptor number. Blood alcohol was at undetectable levels at the time of experimentation.
Conclusions
NMDA receptor sensitivity is decreased on posterior VTA putative dopamine neurons in P rats on a nondependent schedule of alcohol consumption. Mechanisms underlying increased spontaneous dopamine neuron activity may be independent of changes in NMDA receptor function. Decreased NMDA receptor sensitivity may precede the development of dependence.
Keywords: Ventral Tegmental Area, NMDA Receptors, Alcohol-Preferring (P) Rats, Nondependent Alcohol Consumption, Nonlinear Mixed Effect Model
INTRODUCTION
The mesocorticolimbic dopamine system is involved with the acquisition of survival-promoting behaviors. The ventral tegmental area (VTA) contains the cell bodies of dopamine neurons which project to the prefrontal cortex, hippocampus, amygdala, and nucleus accumbens (NAc). When a rodent accesses survival-promoting stimuli (such as food or water). dopamine neuronal activity within the VTA is stimulated, leading to increased dopamine release across the mesocorticolimbic system (Kwint and de Vries, 1997; Pfaus et al., 1995; Yoshida et al., 1992; Yun et al., 2004). This phenomenon is instrumental for increasing the probability of the rodent repeating a sequence of behaviors that allows for further access to survival-promoting stimuli (i.e. adaptive behaviors).
The mesocorticolimbic dopamine system is also involved in the acquisition of maladaptive behaviors. Alcohol, a stimulus not considered survival-promoting, “hijacks” the mesocorticolimbic system by increasing neuronal activity in the VTA (Appel et al., 2003; Brodie et al., 1999; Gessa et al., 1985) and thus dopamine release in the NAc (Di Chiara and Imperato, 1985; Weiss et al., 1993). The stimulation of VTA dopamine neurons by alcohol may be a direct consequence of its pharmacological effects because application of alcohol to dissociated VTA dopamine neurons, an vitro preparation devoid of synaptic inputs, similarly increases their activation (Brodie et al., 1999). Recurrent activation of the mesocorticolimbic system by alcohol promotes sensitization to its rewarding properties (Ding et al., 2009; Franklin et al., 2009), increasing the motivation to sustain alcohol consumption. Thus, the brain may attribute a value to alcohol that is similar to food or water, resulting in the acquisition of alcoholism, presumed to be a maladaptive behavior that distracts one from completing adaptive behaviors.
Selectively-bred alcohol-preferring (P) rats are a model of the “at-risk” phenotype. Innate abnormalities in neurotransmission and the response of the mesocorticolimbic dopamine system to alcohol may promote consumption in these rats. For example, repeated alcohol exposure results in increased dopamine release in the NAc of P rats compared to selectively-bred non-preferring rats (Smith and Weiss, 1999) and basal dopamine levels are elevated in the NAc of drinking P rats compared to alcohol-naïve P rats following eight weeks of nondependent voluntary consumption (Thielen et al., 2004). The increase in accumbal dopamine levels co-occurs with an increase in the number of spontaneously active putative dopamine neurons in the posterior region of the VTA (Morzorati et al., 2010). These observations complement each other and suggest that neuroadaptive changes within the mesocorticolimbic dopamine system of P rats accompany nondependent voluntary alcohol consumption.
An increase in posterior VTA putative dopamine neuron activity following nondependent alcohol consumption may be a consequence of sensitization to N-methyl-D-aspartic acid (NMDA) mediated glutamatergic neurotransmission. NMDA applied to the VTA increases dopamine release within the mesocorticolimbic system (Karreman and Westerink, 1996; Suaud-Chagny et al., 1992; Westerink et al., 1996). NMDA receptor activation converts the relatively stable firing of dopamine neurons into burst firing (Overton and Clark, 1992), which is especially efficacious in increasing dopamine release within dopaminergic projection regions (Floresco et al., 2003; Gonon, 1988; Sombers et al., 2009). NMDA receptors on VTA dopamine neurons may therefore vitally mediate mesocorticolimbic dopaminergic activity, and concurrently, the acquisition of behavioral reinforcement (Zellner et al., 2009; Zweifel et al., 2009).
Others have observed that NMDA and non-NMDA glutamate receptor up-regulation in the VTA occurs as a consequence of dependence-inducing chronic alcohol exposure (Ortiz et al., 1995; Stuber et al., 2008). It therefore seems reasonable to hypothesize that spontaneously active dopamine neurons in this region may exhibit increased sensitivity to glutamate neurotransmission. The current study tested the hypothesis that nondependent chronic alcohol consumption increases NMDA receptor function on posterior VTA putative dopamine neurons in P rats. Such increased receptor function would allow for enhanced stimulation of these neurons, perhaps contributing to the mechanisms responsible for an increase in the number of dopamine neurons that are spontaneously active. Dopamine neurons from the posterior VTA were targeted because this region is directly susceptible to the reinforcing effects of alcohol (Rodd et al., 2004) and innervates the NAc (Ikemoto, 2007). NMDA was microiontophoretically applied to posterior VTA putative dopamine neurons in vivo in the absence of anesthesia, and neuronal responses were recorded and analyzed. To examine how chronic nondependent alcohol consumption may affect NMDA receptor sensitivity, current-response curves were constructed from NMDA-induced neuronal responses. Values for EC50 (the effective current that produces a half maximal excitatory response) and Emax (the maximal effect) as well as CDB (the current at which depolarization block occurs), were compared between alcohol naïve P rats and chronic alcohol drinking P rats.
MATERIALS AND METHODS
Animals
Adult female alcohol-preferring (P) rats (generations 63–67) were obtained from the Indiana University Alcohol Research Center. Female P rats were used because they have been used in previous experiments conducted by this laboratory (Morzorati et al., 2010; Engleman et al., 2011) and their continued use allows for comparison of results. The rats were acclimated to the facilities for three weeks before being individually-housed in 11.0” × 8.4” cages. The animal facilities are fully accredited by the Association for Assessment and Accreditation of Laboratory Animal Care. All research protocols were pre-approved by the Institutional Animal Care and Use Committee in strict compliance with the guidelines of the National Institutes of Health Guide for the Care and Use of Laboratory Animals (Publication No. 80–23, revised 1996).
Alcohol consumption protocol
Rats were randomly assigned to alcohol naïve (control) or chronic alcohol drinking (24-hour access for 8 weeks) groups. Rat chow and water were available ad libitum. Rats in the chronic drinking group were acclimated to a 15% alcohol/water solution (v/v) for four days, after which alcohol solution and water intakes and rat weights were recorded three times per week. Drinking scores were computed by converting alcohol solution intake to average grams of ethanol consumed per kilogram of body weight per day (g/kg/day). Weekly averages for body weight, ethanol consumption and percent of ethanol to total fluid consumed were then computed. Experiments specifically designed to monitor alcohol intake reported that with free choice access to alcohol and water, adult female P rats drink in bouts, primarily during the dark cycle (Bell et al., 2006), and can achieve blood alcohol concentrations (BACs) of 90 mg% per bout (cf. Murphy et al., 1986). For the duration of their stay, all rats were acclimated to handling to reduce stress because handling-related stress is known to affect dopaminergic activity in rats (Enrico et al., 1998). Four hours prior to electrophysiology experiments, chronically drinking rats were denied access to alcohol to ensure that blood alcohol levels, if present, would diminish to non-detectable levels. In addition, vaginal smears were obtained to determine the stage of estrous. Rats were used only when circulating levels of estrogen were low since dopaminergic neuronal activity is modulated by estrogen (Chiodo and Caggiula, 1983). Thus, rats were not used if they were cycling through proestrous, a stage in which blood estrogen is high and the vaginal smear is predominated by nucleated epithelial cells, while cornified cells and leukocytes are low in number (Hubscher et al., 2005). During the last week of drinking and prior to experimentation, the drinking rats were observed for physical signs of dependence (stiff tail, hyper-reactivity, tremors, aimless locomotion or stereotypical body movements; Waller et al., 1982).
Surgery
General anesthesia was induced and maintained during the surgical procedure with an isoflurane/oxygen/nitrogen mixture. All incision sites and pressure points were infiltrated with a long-acting local anesthetic (Carbocaine-V, 2% mepivacaine hydrochloride). The right femoral vein and artery were cannulated for administration of fluids and blood withdrawals. The trachea was intubated with polyethylene tubing (PE 260) via an incision on the ventral neck and the rat was positioned in a stereotaxic apparatus. The skull was leveled, a large craniotomy was made overlying the right posterior VTA and the meninges were carefully retracted. Indifferent and ground screws were threaded into the contralateral skull.
After surgery, a paralyzing agent (gallamine triethiodide, 25 mg/kg, i.v.) was administered to produce complete paralysis of all skeletal muscles. Paralyzed – rather than anesthetized – rats were chosen because general anesthesia was shown to directly modify dopaminergic neuronal activity (Kelland et al., 1989; Windels and Kiyatkin, 2006). It is believed the rats did not experience pain or stress because the baseline firing rates reported here are similar to those reported at baseline in presumably pain- and stress-free freely moving rats (Anstrom and Woodward, 2005) and are dissimilar to those in rats subjected to acute restraint stress (Anstrom and Woodward, 2005). It is believed that ethanol drinking history did not have differential effects on pain or stress perception since baseline firing frequencies and measurements of burst activity did not differ in alcohol naïve and chronically drinking P rats. Once gallamine was administered, isoflurane was discontinued and the tracheal cannula was connected to a volume-controlled respirator (Model 683 Small Animal Ventilator, Harvard Apparatus, Holliston, MA) and the oxygen/nitrogen mixture.
The rat recovered from anesthesia for 30 minutes before extracellular recording commenced. An ophthalmic ointment (LubriFresh) was applied to the eyes to prevent desiccation. Body temperature was maintained at 37° C with a homeothermic heating system (Model 50-7053-R, Harvard Apparatus, Holliston, MA). Arterial blood samples were withdrawn from all rats prior to experimentation and regularly throughout to monitor pCO2 and pO2. Adjustments to respiratory rate and/or the flow of nitrogen and oxygen were made as needed to keep pCO2 and pO2 within normal limits. Ringer’s lactate was periodically administered subcutaneously to maintain tissue hydration. Gallamine was administered at 30-minute intervals to ensure immobility. Carbocaine-V was infiltrated into all pressure points and incision sites every 3 h to control pain. The surface of the brain was kept moist with warm artificial cerebrospinal fluid.
To ensure that the presence of blood alcohol did not confound the results of the drinking rats, BACs were determined using an Analox Analyzer (Model GL5, Analox Instruments USA, Lumenburg, MA) just prior to electrophysiological recording. Alcohol concentrations in plasma samples were determined by measuring oxygen uptake generated by the oxidation of ethanol to acetaldehyde and hydrogen peroxide by ethanol oxidase. Demonstrable alcohol content was criterion for discarding data collected from that particular rat. No data was eliminated from analysis because of detectable BACs.
Recording and microiontophoresis
Electrodes were constructed based upon the “piggy-back” design (Stone, 1985). Single- and four-barrel glass pipettes (A–M Systems, Sequim, WA) were pulled with a Narishige pipette puller (Model PE-2), yielding a hip-to-tip length of 12–13 mm so that the upper cortex was not damaged during electrode insertion into the posterior VTA. Recording electrodes were created by breaking the tip of the single-barrel pipette to 2 µm diameter and were filled with 2 M NaCl (in vitro impedances of 2.4 – 4.0 MΩ at 135 Hz). Signals from the recording electrode and indifferent screw were differentially amplified and filtered with a band-pass of 300–10,000 Hz, displayed on an oscilloscope and monitored on an audio amplifier. The signals were also routed through an analog-to-digital converter (Cambridge Electronic Design, [CED], Cambridge, England) interfaced to a PC running Spike2 software (CED), displayed on the computer monitor and selected for analysis using a template-matching routine. The drug-delivery electrode was created by breaking the tip of the 4-barrel glass pipette to a diameter of 4–6 µm. The barrels of the drug-delivery electrode were filled with NMDA (20 mM in distilled water, pH 8.0, Sigma-Aldrich, St. Louis, MO), saline (adjusted to pH 8.0), or (2R)-amino-5-phosphonovaleric acid (AP5; 25 mM in distilled water, pH 8.0; Sigma-Aldrich, St. Louis, MO). One of the drug electrode barrels was filled with saline and used for current balancing. Drug-barrel impedances ranged between 30–90 MΩ while impedances for the current-balancing barrel were lower (< 30 MΩ). Drug ejections were controlled by a Dagan 6400 microiontophoresis system (Dagan Corporation, Minneapolis, MN). Retaining currents of −10 nanoamperes (nA) were applied to drug barrels and pH-adjusted saline barrels to reduce diffusion into the extracellular space.
The exposed skull was marked with a grid delineating the rostrocaudal and mediolateral extents of the posterior VTA (from bregma, posterior: 5.3 – 6.3 mm; from midline, lateral: 1.3–1.7 mm; Paxinos and Watson, 1998). Starting at the brain’s dorsal surface, microiontophoretic electrodes were lowered into the posterior VTA (ventral: 7.7 – 9.8 mm, 6° angle) with a micromanipulator mounted onto the stereotaxic frame. Electrode descents were made slowly to ensure that spontaneously active neurons of various firing frequencies were recorded. Putative dopamine neurons were identified by analyzing action potentials and comparing them to previously-established criteria: (1) broad (≥ 2.5 ms), triphasic (+\−\+) action potential waveforms; (2) a prominent “notch” on the initial rising phase; and (3) a slow, irregular firing pattern (Grace and Bunney, 1983). Previous observations indicate that neurons recorded from the posterior VTA with these characteristics respond to the D2-like receptor agonist quinpirole by decreasing their firing rate and respond to the D2-like receptor antagonist eticlopride by increasing their firing rate (Morzorati and Marunde, 2006.) It should be noted that the efficacy in identifying dopamine neurons based upon their waveform characteristics and responsiveness to D2 receptor agonists has been both supported and refuted in studies from other laboratories (Grace and Bunney, 1983; Grace and Onn, 1989; Johnson and North, 1992; Margolis et al., 2006, 2008).
To eject NMDA onto a neuron, ascending currents of −2 nA to −14 nA were incrementally applied in 60 s periods to the drug barrel (e.g., −4 nA was applied to the NMDA barrel for 60 s, resulting in ejection of NMDA into the extracellular space and constituting a −4 nA “dose”). If depolarization block occurred, higher currents were not ejected because depolarization block is an indicator that the maximum excitatory response of a cell has been reached. Depolarization block follows a period of sustained excitation and is typified by a sudden and marked decrease in firing frequency and burst activity during NMDA ejection. Each ejection period was separated by 30 s to allow the neuron to return to baseline firing. Due to a phenomenon known as the “warm-up effect” (Overton and Clark, 1992; Stone, 1985), each current was repeated until two successive, similar neuronal responses were attained.
To ensure NMDA-induced changes in firing frequency were receptor-mediated, a separate set of experiments was conducted in which AP5 (a competitive NMDA receptor antagonist) was ejected at currents of −3 to −5 nA concurrently with ejections of NMDA at −4 to −8 nA for 60 s periods. AP5 ejection currents varied between neurons to avoid significant changes in baseline firing frequency. To ensure that changes in a neuron’s activity during NMDA ejections were not the result of H+ ejection or artificial stimulation by currents applied to the drug barrel, saline (pH 8.0) was ejected for 60 s periods in incremental currents from −6 nA to −12 nA. Each ejection period was interspersed by 30 s intervals, mimicking the NMDA ejection protocol.
Histology
At the end of the experiment, an electrode filled with 1% Chicago Sky Blue (Sigma-Aldrich, St. Louis, MO) in 2 M NaCl was lowered into the recording area until putative dopamine neuron action potentials were encountered. The rat was deeply anesthetized with isoflurane and dye was ejected (20 µA for 15 minutes). The rat was decapitated and the brain quickly removed and frozen in isopentane. Frozen brains were sliced (40 µm thickness) into consecutive coronal sections at the site of recording and were stained with cresyl violet. The location of the dye deposits and any marks made by the electrode penetrations were determined using light microscopy. The posterior VTA is located medial to the substantia nigra pars compacta which also contains dopaminergic neurons; therefore, if stained brain sections did not conclusively indicate that the posterior VTA had been targeted for recording, data obtained from that rat were not included in analyses. Data from 2 rats were not included in the analysis because of poor electrode placements.
Analyses of firing frequency, burst activity, EC50, Emax, CDB
Recordings of putative dopamine neuron action potentials were analyzed using Spike2 software (version 6.14, CED). Artifactual spikes (such as those occurring from discharge of GABA interneurons or current artifacts from equipment) were few in number and were manually removed from recordings by a trained technician. Dopamine neuronal responses to NMDA ejections were quantified in domains of firing frequency, number of bursts, and spikes occurring in bursts. To determine baseline neuronal activity, the mean firing frequency (in Hz) for 60 s prior to the first NMDA ejection was used; the total number of bursts, spikes occurring in bursts, and spikes occurring outside of bursts during this period were used to determine baseline measures of burst activity. Due to the previously-described “warm-up effect,” two consistent responses to each ejection current were obtained in individual neurons, and the average of these two responses was used to compute current-response curves. Firing frequency and the measures of burst activity were extracted with the aid of a readily-available Spike2 script (Burst_v1.31; CED). A burst onset began when two action potentials (“spikes”) discharged within 80 ms of each other, and the burst terminated when two action potential discharges were separated by 160 ms (Grace and Bunney, 1984). The conglomeration of spikes occurring between burst onset and termination constituted a single burst event. Discrete burst events were recorded into a separate channel at the time that burst onset occurred and counted to obtain number of bursts. Spikes occurring within burst events and spikes occurring outside of burst events were also displayed in separate channels and were counted.
To examine the general null hypotheses that there were no group differences for firing frequency and burst activity, current-response curves for chronic alcohol drinking and alcohol naïve P rats were initially compared using nonlinear mixed effects models (Vonesh and Carter, 1992). These models allow accurate estimation of population parameters from limited sample sizes and correct for variance heterogeneity often accompanying cumulative dose-response data (Thorin et al., 2010). Current response curves from 0 to −14 nA were constructed for each group of rats and group-by-curve interactions were fitted using the Linear and Nonlinear Mixed Effects Models package for R (Pinheiro and Bates, 2000), treating individual neurons as a random effect to account for within-group variability in neuronal responses. Cubic polynomial functions were used for modeling firing frequency, number of bursts and spikes occurring in bursts. This provided sufficient flexibility to adequately describe each measure. Group curves for each measure of neuronal activity were compared with F-tests.
Once significant F test differences were realized for group current-response curves, values for EC50 (the effective current needed to produce a half maximal excitatory response) and Emax (the maximal excitatory effect) were obtained from the polynomial modeled current-response curves for firing frequency, number of bursts and spikes occurring in bursts. For determining EC50 values, the data were normalized. For this, baseline values were subtracted from the responses after each ejection current; current-responses were converted to percent maximum response and curves were constructed based on a nonlinear mixed effects model (Thorin et al., 2010). Group EC50 and Emax estimates were compared by examining 95% confidence intervals.
Values for CDB (the current at which depolarization block occurred) were obtained from the raw data and only those neurons whose firing frequency had ceased for most of the ejection period (> 30 s) were used. Consequently, the number of neurons used to compare CDB is smaller than the number of neurons used to construct the current-response curves (14 and 10 neurons used to determine CDB versus 23 and 19 neurons used to construct the current-response curves, for alcohol naïve and chronic alcohol drinking rats, respectively).
Two-sample t-tests were used to compare group means for rat body weights, action potential durations, recoding depths, baseline firing frequencies, baseline number of bursts, baseline spikes occurring in bursts, baseline spikes occurring outside of bursts and CDB. Equality of variances was first evaluated using Levene’s test; if variances were unequal, the Welch correction was applied. Ethanol consumption and percent ethanol preference were evaluated over the 8-week drinking protocol by examining 95% confidence intervals. For all statistical analyses, α = 0.05 was the cutoff for significance
RESULTS
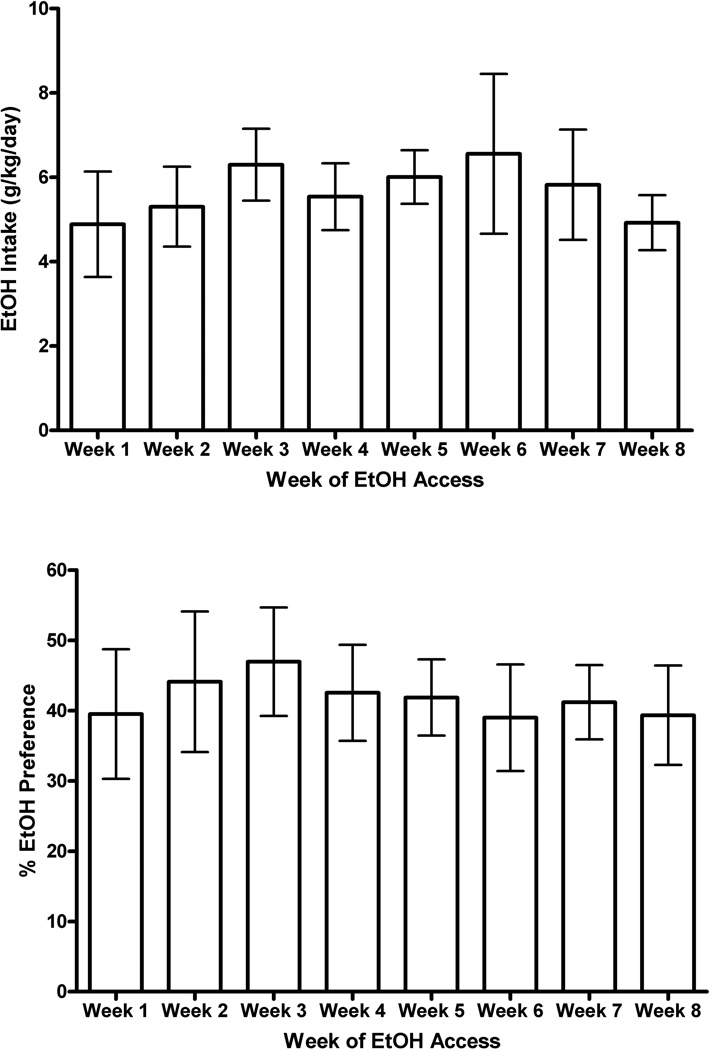
Data were obtained from 16 alcohol naïve P rats and 12 chronic alcohol drinking P rats (165–172 days old) in which the microiontophoretic electrode was clearly in the posterior VTA (Figure 1). Body weights at the time of the electrophysiological experiments were similar between the rat groups (mean ± SEM; chronic drinking rats: 309.3 ± 9.5 g, alcohol naïve rats: 322.6 ± 4.8 g). When examining 95% confidence intervals, weekly ethanol consumption and percent ethanol preference were consistent for chronic drinking P rats across the 8-week drinking protocol. Ethanol consumption averaged 5.8 ± 0.2 g/kg/day (range 4.3 – 6.5 g/kg/day) and percent alcohol preference averaged 41.1 + 2.7% (Figure 2). The chronically drinking P rats were not dependent on alcohol since they did not exhibit overt signs of alcohol dependence or withdrawal, consistent with previous observations employing this drinking protocol (Thielen et al., 2004).
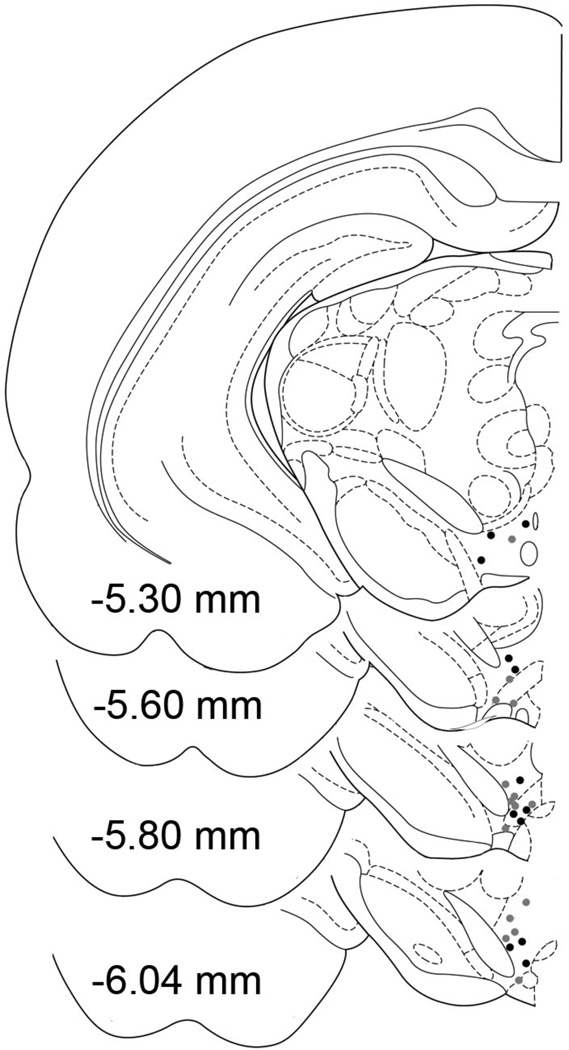
Figure 1.
Anteroposterior hemi-coronal sections illustrating the locations of the microiontophoretic electrodes in the posterior VTA of alcohol naïve (gray circles) and nondependent chronic alcohol drinking (black circles) P rats. Some overlapping electrode placements are not shown. Anteroposterior plates with reference to bregma (Paxinos and Watson, 1998).
Figure 2.
Weekly ethanol intake (top panel) and percent ethanol preference (bottom panel) averaged for the 12 nondependent chronic alcohol drinking P rats across the 8-week drinking protocol. Bars represent means ± 95% confidence intervals. As evidenced by the confidence intervals, ethanol intakes and preferences were consistent over the 8 weeks of drinking.
Microiontophoretic electrode recording depths ranged between 7.6 – 9.0 mm (8.3 ± 0.6) below the surface of the brain. There were no significant group differences in action potential durations (chronic alcohol drinking rats 3.8 + 0.1 ms, alcohol naïve rats 3.8 + 0.1 ms), baseline firing frequencies (chronic alcohol drinking rats 3.7 ± 0.3 Hz, alcohol naïve rats 3.2 ± 0.4 Hz), baseline number of bursts (chronic alcohol drinking rats 13 ± 3 bursts, alcohol naïve rats 11 ± 3 bursts), baseline spikes occurring in bursts (chronic alcohol drinking rats 48 ± 11 spikes, alcohol naïve rats 40 ± 14 spikes) or baseline spikes occurring outside of bursts. These characteristics are similar to those of putative dopamine neurons observed in previous studies (Morzorati and Marunde, 2006; Morzorati et al., 2010).
NMDA ejections elicited increases in firing frequency in putative dopamine neurons from the posterior VTA of chronic alcohol drinking rats (19 neurons) and alcohol naïve P rats (23 neurons). These responses were current-dependent, with ascending ejection currents correspondingly resulting in higher firing frequencies (Figure 3a). Firing frequency for each putative dopamine neuron increased rapidly in response to NMDA ejections, followed by a rapid return to baseline firing rate when the ejection current was terminated. The appearance of depolarization block at higher ejection currents signified that the maximum response of the neuron had been reached. To verify that observed increases in firing frequency were receptor-mediated, NMDA was ejected onto putative dopamine neurons while concurrently ejecting AP5, a competitive NMDA receptor antagonist. Posterior VTA dopamine neurons were sampled from chronically drinking and alcohol naïve rats. In the eight neurons tested with this procedure (4 neurons in each group of rats), concurrent ejection of AP5 blocked NMDA-induced increases in firing frequency (Figure 3b). Microiontophoretic application of saline (pH 8.0) onto putative dopamine neurons (n = 9) in doses up to −12 nA did not induce changes in firing frequency.
Figure 3.
Prototypical rate histograms (20 s mean frequency bins) of posterior VTA putative dopamine neurons in separate nondependent alcohol drinking P rats for (a) cumulative NMDA current-responses, and (b) concurrent NMDA and AP5 ejections. (a) NMDA produced current-dependent increases in firing frequency. Occurrence of depolarization block was exemplified by a rapid decrease in firing frequency immediately following the peak response during the −10 nA ejection period. Horizontal bars indicate NMDA ejection periods accompanied by ejection currents (−2, −4, −6, −8, −10 nA). (b) NMDA (−6 nA) increased firing frequency which stabilized during the 3rd and 4th ejections. Concomitant ejection of AP5 (a competitive NMDA receptor antagonist) at −5 nA blocked the NMDA-induced increases in firing frequency. When the AP5 current was terminated, NMDA ejection (−6 nA) again increased firing frequency which stabilized during the 2nd and 3rd ejections. Horizontal bars indicate NMDA (black bars) and AP5 (gray bar) ejection periods and currents.
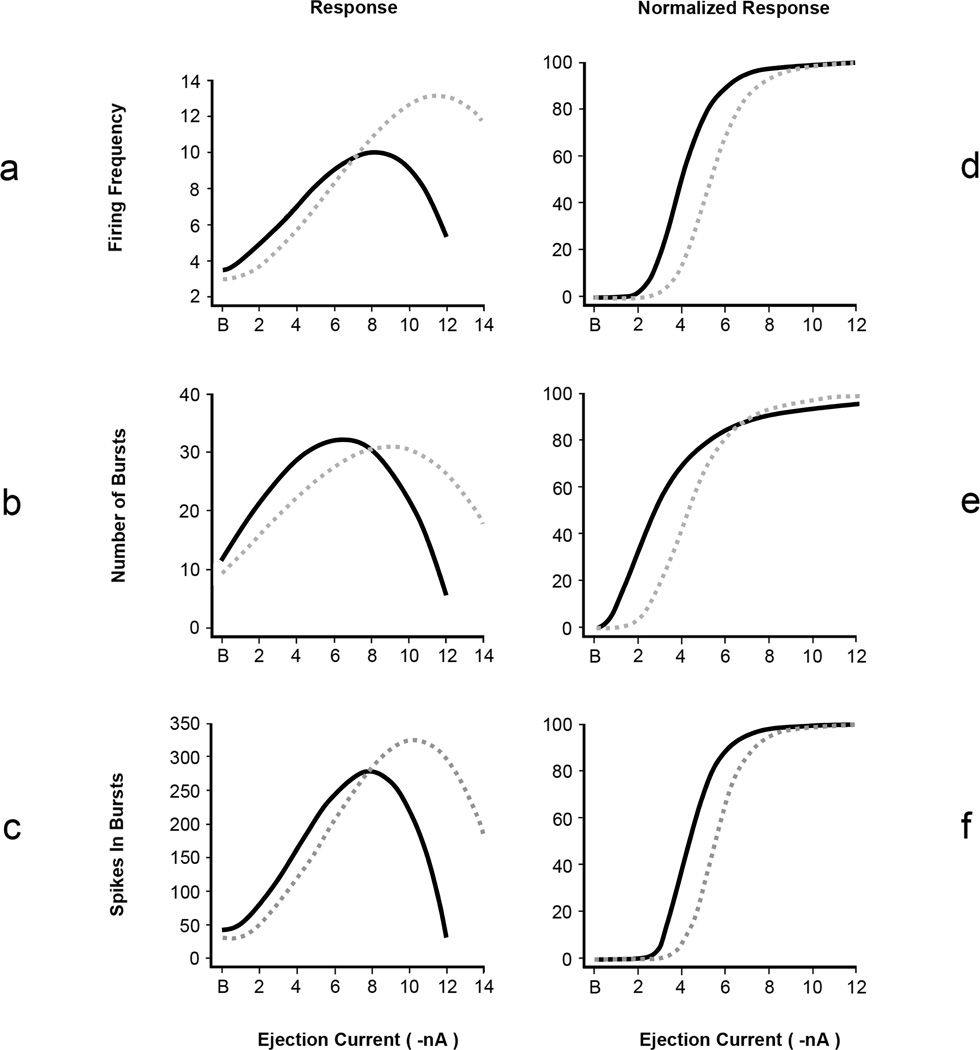
Using a nonlinear mixed effect model, it was initially demonstrated that current-response curves for firing frequency (F [4, 198] = 11.0, p < 0.0001), number of bursts (F [4, 198] = 3.6, p = 0.0076) and spikes occurring in bursts (F [4, 198] = 3.6, p = 0.007) were significantly different between nondependent chronic alcohol drinking and alcohol naïve P rats (Figure 4 a, b, c). Spikes occurring outside of bursts were not different between the groups of rats (p > 0.05, data not shown). To determine which characteristics of the curves drove these differences, EC50 and Emax were calculated. As determined by examining 95% confidence intervals, EC50 estimates for chronic alcohol drinking P rats were significantly higher than for alcohol naïve P rats for firing frequency, number of bursts and spikes occurring in bursts (Table 1, Figure 4 d, e, f). Examining 95% confidence intervals indicated there were no differences in Emax values between the rat groups for all measures of neuronal activity (Table 1, Figure 4 a, b, c). Onset of depolarization block is indicated by the downward deflection succeeding maximal responses in the current-response curves in Figure 4a. Two-sample t-tests indicated that depolarization block occurred at significantly higher ejection currents in neurons from chronic alcohol drinking P rats (CDB, Table 1). Graphs showing the raw data used to generate the fitted curves in Figure 4 can be found in the online version of the article (see Supporting Information following the references).
Figure 4.
Group current-response curves of posterior VTA putative dopamine neurons from alcohol naïve P rats (solid black lines; 23 neurons) and nondependent chronic alcohol drinking P rats (dashed gray lines; 19 neurons) at baseline (B, 0 nA) and in response to microiontophoretic NMDA ejections (−2 to −14 nA). The curves were fitted with a cubic polynomial function and tested for statistical significance using F tests generated by a nonlinear mixed effect model. In general, the curves were significantly different between the groups of rats for all neuronal activity: (a) firing frequency (p < 0.0001), (b) number of bursts (p = 0.0076) and (c) spikes occurring in bursts (p = 0.007). Emax was not different between rat groups for any measure of neuronal activity. CDB was significantly (p=0.04) greater in chronic alcohol drinking P rats. Values for EC50 were determined from normalized current-response curves (d, e, f) and were significantly higher (p ≤ 0.05) in chronic alcohol drinking P rats for all measures of neuronal activity.
Table 1.
Results of analyses comparing EC50, Emax and CDB in alcohol naïve and nondependent alcohol drinking P rats following NMDA microiontophroesis.
| Alcohol Naïve P Rats | Alcohol Drinking P Rats | |
|---|---|---|
| Firing Frequency | ||
| EC50 (nA) | ||
| Mean (± SEM) | 4.1 ± 0.3 | 5.4 ± 0.3 |
| 95% CI (Lower, Upper) | 3.5, 4.7 | 4.8, 5.9 * |
| Emax (spikes/sec, Hz) | ||
| Mean (± SEM) | 10.1 ± 0.7 | 13.3 ± 1.4 |
| 95% CI (Lower, Upper) | 8.7, 11.6 | 10.5, 16.0 |
| CDB (nA) | ||
| Mean (± SEM) | 8.6 ± 0.4 | 9.6 ± 0.3 * |
| Number of Bursts | ||
| EC50 (nA) | ||
| Mean | 2.7 ± 0.2 | 4.2 ± 0.4 |
| 95% CI | 2.3, 3.1 | 3.3, 5.1 * |
| Emax (bursts) | ||
| Mean | 32.4 ± 2.4 | 31.3 ± 2.9 |
| 95% CI (Upper, Lower) | 27.7, 37.1 | 25.5, 37.0 |
| Number of Spikes in Bursts | ||
| EC50 (nA) | ||
| Mean | 4.4 ± 0.3 | 5.6 ± 0.3 |
| 95% CI | 3.8, 5.0 | 5.0, 6.2 * |
| Emax (spikes) | ||
| Mean | 281.0 ± 30.7 | 327.4 ± 44.0 |
| 95% CI (Upper, Lower) | 219.6, 342,3 | 239.4, 415.3 |
p ≤ 0.05; SEM = standard error of the mean; CI = confidence intervals; spikes = action potentials; EC50 = the effective current needed to produce a half maximal excitatory response to NMDA; Emax = the maximal excitatory effect produced by NMDA; CDB = current at which depolarization block occurred. EC50 and Emax were analyzed using a nonlinear mixed effects model (see text) and compared by examining 95% confidence intervals. CDB values were obtained from the raw data and compared using t-tests.
DISCUSSION
To our knowledge, this is the first study to assess alterations of glutamatergic transmission in the mesocorticolimbic system resulting from nondependent alcohol consumption. The differences in current-response curves between putative dopamine neurons from chronic drinking and alcohol naïve P rats suggests differences in NMDA receptor function following eight weeks of nondependent voluntary consumption. Current-response curves for firing frequency, number of bursts and spikes in bursts were shifted to the right for putative dopamine neurons from chronic drinking rats, as evidenced by higher EC50 values. This suggests that larger doses (ejection currents) of NMDA were needed in chronic drinking P rats to elicit increases in firing frequency and burst activity comparable to those elicited in alcohol naïve P rats. In addition, putative dopamine neurons from chronic drinking rats exhibited resistance to depolarization block in response to constant NMDA-mediated depolarization, as evidenced by higher CDB values. Values for Emax did not differ between alcohol drinking and naive P rats, suggesting that the number of NMDA receptors located on recorded neurons did not differ between groups. Taken together, these results are consistent with decreased affinity of NMDA receptors on posterior VTA putative dopamine neurons for ligand in chronically drinking P rats but no difference in NMDA receptor number between drinking and alcohol naïve P rats.
Glutamate release onto VTA dopamine neurons activates both NMDA and AMPA (α-amino-3-hydroxy-5-methylisoxazole-4-proprionic acid) receptors (Chen et al., 2001; Wang and French, 1993). AMPA receptor activation allows for the influx of Na+ ions into the neuron, resulting in depolarization and the generation of fast excitatory signals. In order for glutamate to induce sustained firing in dopaminergic neurons, NMDA receptors must be activated (Deister et al., 2009). Under resting conditions, NMDA receptor channel pores are occluded by Mg2+ ions. Sufficient depolarization caused by activation of AMPA receptors removes the Mg2+ ion block and allows glutamate to activate NMDA receptors. This, along with activation of NMDA receptors by glycine, a necessary co-agonist, causes the influx of cations, especially Na+ and Ca2+ ions, and the generation of a slow excitatory post-synaptic potential (EPSP). In dopamine neurons, Na+-dependent spikes ride this slow EPSP, resulting in a burst of action potentials that are dependent on the voltage-gated characteristics of the NMDA receptor (Deister et al., 2009). Intense, successive depolarizations cause dopamine neurons to enter depolarization block, a state in which the membrane potential is sustained above the threshold for action potential generation, leading to a sudden and marked decrease in firing frequency and burst activity. Depolarization block may be mediated by inactivation of Na+ ion channels and/or activation of Ca2+-dependent K+ ion channels and generation of a large magnitude, long duration hyperpolarization (Blythe et al., 2009; Johnson et al., 1992; Kim et al., 2004).
A decrease in NMDA receptor function may explain the smaller increases (larger EC50 values) in firing frequency and burst activity of posterior VTA putative dopamine neurons to moderate doses of NMDA in chronic drinking P rats. A number of factors can contribute to alcohol’s reduction of NMDA receptor function, including alterations in glutamate release from afferents (Xiao et al., 2008), extracellular Mg2+ and glycine concentrations (Martin et al., 1991; Rabe and Tabakoff, 1990), intracellular Ca2+ concentrations (Dildy-Mayfield and Leslie, 1991), receptor subunit composition (Jin and Woodward, 2006; Ortiz et al., 1995) and/or posttranslational modifications (Ferrani-Kile et al., 2003; Miyakawa et al., 1997). Reduced function of Ca2+-dependent K+ ion channels may explain the resistance to depolarization block in chronic nondependent alcohol drinking P rats (lower CDB) and similar maximal responses for firing frequency and burst activity (similar Emax values) in drinking and naïve rats. Diminished hyperpolarization produced by Ca2+-dependent K+ ion channels would allow for sustained burst activity at high concentrations of glutamate in chronically drinking P rats while burst activity in alcohol naïve rats would be terminated by depolarization block. Only with larger doses of NMDA is hyperpolarization produced by Ca2+-dependent K+ ion channels sufficient for posterior VTA putative dopamine neurons in chronically drinking rats to enter depolarization block. Burst firing by dopamine neurons is thought to represent a key component of the reward circuitry. Dopamine neuron burst activity increases dopamine release in projection areas (Gonon, 1988) and is associated with stimuli that predict the delivery of reward (Schultz, 1998). Thus, the prolongation of burst activity in chronic drinking P rats would reinforce alcohol consumption and could lead to the development of dependence.
NMDA receptor function in VTA dopamine neurons was inhibited with acute alcohol administration (Allgaier et al, 1999). Chronic dependence-inducing alcohol exposure resulted in a compensatory up-regulation of VTA NMDA receptors (Ortiz et al., 1995), perhaps in response to repeated inhibition. This leads to increased NMDA receptor function and a state of excessive activation when alcohol is suddenly withdrawn (Hendricson et al., 2007; Lack et al., 2007). When behavioral signs of withdrawal ceased, NMDA receptor function on VTA dopamine neurons decreased (Bailey et al., 1998). Since the increase in NMDA receptor function during withdrawal was transient and the decrease in NMDA receptor function did not correlate with signs of withdrawal, the latter may be related to more prolonged neuroadaptive changes in response to alcohol. With respect to Ca2+-dependent K+ ion channels, Hopf et al. (2007) reported that their function was decreased in VTA dopamine neurons following cessation of withdrawal symptoms. Again, the changes in Ca2+-dependent K+ ion channels may be related to long-lasting neuroadaptive changes in response to alcohol.
The results of the present study do not support our hypothesis which stated that nondependent chronic alcohol consumption by P rats increases the function of NMDA receptors on posterior VTA dopamine neurons. This hypothesis was posited because we recently reported that the number of spontaneously active putative dopamine neurons in the posterior VTA increased with nondependent alcohol consumption in P rats (Morzorati et al., 2010). Increased functioning of NMDA receptors could allow for enhanced dopamine neuron stimulation, thereby contributing to the mechanisms underlying an increase in spontaneous activity. However, given the present findings, it is possible that the mechanisms contributing to increased spontaneous activity following alcohol consumption may be independent of changes in NMDA receptor function.
In general, the activity of VTA dopamine neurons is orchestrated by a balance between activation of excitatory and inhibitory receptors. Approximately half of dopamine neurons are inactive because their membrane potentials are hyperpolarized (Chiodo, 1988). The excitatory/inhibitory balance may swing towards more excitatory influences, fewer inhibitory influences or a combination of both that favors moving the membrane potential towards threshold for firing. In this way, dopamine neurons that were once quiescent now fire spontaneously. The following illustrate possible alterations in excitatory and inhibitory influences that may result in an increased number of spontaneously active VTA dopamine neurons.
Increased excitatory influences
NMDA and AMPA receptors coexist on VTA dopamine neurons and their ratio is of particular interest in modulating dopamine neuron activity and enhancing the reinforcing effects of addictive drugs such as cocaine and morphine (Carlezon and Nestler, 2002). The ratio of AMPA to NMDA receptors was increased in VTA dopamine neurons of rats following chronic voluntary consumption of alcohol (Stuber et al., 2008). This was indicated by an increased ratio of evoked AMPA to NMDA excitatory postsynaptic currents. Gao et al. (2010) reported that nicotine exposure also increased the AMPA to NMDA receptor ratio on VTA dopamine neurons as well as increased the number of active dopamine neurons. In the same way, chronic nondependent alcohol drinking may have increased the AMPA to NMDA receptor ratio in P rats, resulting in conversion of silent to active dopamine neurons.
Decreased inhibitory influences
Floresco et al. (2003) reported that separate afferent pathways to the VTA differentially regulate the firing properties of dopamine neurons. The ventral pallidum supplies inhibitory GABAergic afferents to the VTA (Wu et al., 1996). Pharmacological inhibition of pallidal afferents increased the number of spontaneously active tegmental dopamine neurons and increased the extracellular levels of dopamine in the NAc (Floresco et al., 2003). These data suggest that activation of GABA receptors contributes to dopamine neuron inactivity. Likewise, in the present study, chronic nondependent alcohol drinking may have suppressed GABAergic input from the ventral pallidum and altered postsynaptic GABA receptors, allowing quiescent dopamine neurons to fire.
In conclusion, decreased NMDA receptor functioning and resistance to depolarization block was observed in posterior VTA putative dopamine neurons from P rats following eight weeks of nondependent voluntary alcohol consumption. These changes may alter integrative properties in VTA dopamine neurons that represent pathological neuroadaptations that succeed chronic consumption and precede the development of dependence.
Supplementary Material
ACKNOWLEDGMENTS
The authors wish to thank Dr. Larry Lumeng for supplying the selectively-bred P rats and Dr. Eric Engleman for helpful commentary. This work was supported by a grant from the National Institute on Alcohol Abuse and Alcoholism: AA12832.
Footnotes
SUPPORTING INFORMATION
Figure 4S: Graphs showing the raw data used to generate the fitted curves in Figure 4 can be found in the online version of this article.
REFERENCES
- Allgaier C, Scheibler P, Muller D, Feuerstein TJ, Illes P. NMDA receptor characterization and subunit expression in rat cultured mesencephalic neurons. Br J Pharmacol. 1999;126:121–130. doi: 10.1038/sj.bjp.0702284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anstrom KK, Woodward DJ. Restraint increases dopaminergic burst firing in awake rats. Neuropsychopharmacology. 2005;30:1832–1840. doi: 10.1038/sj.npp.1300730. [DOI] [PubMed] [Google Scholar]
- Appel SB, Liu Z, McElvain M, Brodie MS. Ethanol excitation of dopaminergic ventral tegmental area neurons is blocked by quinidine. J Pharmacol Exp Ther. 2003;306:437–446. doi: 10.1124/jpet.103.050963. [DOI] [PubMed] [Google Scholar]
- Bailey CP, Manley SJ, Watson WP, Wonnacott S, Molleman A, Little HJ. Chronic ethanol administration alters activity in ventral tegmental area neurons after cessation of withdrawal hyperexcitability. Brain Res. 1998;803:144–152. doi: 10.1016/s0006-8993(98)00654-4. [DOI] [PubMed] [Google Scholar]
- Bell RI, Rodd ZA, Sable HJK, Schultz JA, Hsu CC, Lumeng L, Murphy JM, McBride WJ. Daily patterns of ethanol drinking in peri-adolescent and adult alcohol-preferring (P) rats. Pharmacol Biochem Behav. 2006;83:35–46. doi: 10.1016/j.pbb.2005.12.004. [DOI] [PubMed] [Google Scholar]
- Blythe SN, Wokosin D, Atherton JF, Bevan MD. Cellular mechanisms underlying burst firing in substantia nigra dopamine neurons. J Neurosci. 2009;29:15531–15541. doi: 10.1523/JNEUROSCI.2961-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brodie MS, Pesold C, Appel SB. Ethanol directly excites dopaminergic ventral tegmental area reward neurons. Alcohol Clin Exp Res. 1999;23:1848–1852. [PubMed] [Google Scholar]
- Carlezon WA, Nestler EJ. Elevated levels of GluR1 in the midbrain: a trigger for sensitization to drugs of abuse? Trends Neurosci. 2002;25:610–615. doi: 10.1016/s0166-2236(02)02289-0. [DOI] [PubMed] [Google Scholar]
- Chen L-W, Wei L-C, Lang B, Ju G, Chan YS. Differential expression of AMPA receptor subunits in dopamine neurons of the rat brain: A double immunocytochemical study. Neuroscience. 2001;106:149–160. doi: 10.1016/s0306-4522(01)00255-x. [DOI] [PubMed] [Google Scholar]
- Chiodo LA. Dopamine-containing neurons in the mammalian central nervous system: electrophysiology and pharmacology. Neurosci Biobehav Rev. 1988;12:49–91. doi: 10.1016/s0149-7634(88)80073-3. [DOI] [PubMed] [Google Scholar]
- Chiodo LA, Caggiula AR. Substantia nigra dopamine neurons: alterations in basal discharge rates and autoreceptor sensitivity induced by estrogen. Neuropharmacology. 1983;22:593–599. doi: 10.1016/0028-3908(83)90150-8. [DOI] [PubMed] [Google Scholar]
- Deister CA, Teagarden MA, Wilson CJ, Paladini CA. An intrinsic neuronal oscillator underlies dopaminergic neuron bursting. J Neurosci. 2009;29:15888–15897. doi: 10.1523/JNEUROSCI.4053-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Chiara G, Imperato A. Ethanol preferentially stimulates dopamine release in the nucleus accumbens of freely moving rats. Eur J Pharmacol. 1985;115:131–132. doi: 10.1016/0014-2999(85)90598-9. [DOI] [PubMed] [Google Scholar]
- Dildy-Mayfield JE, Leslie SW. Mechanisms of inhibition of N-methyl-D-aspartate-stimulated increases in free intracellular Ca2+ concentration by ethanol. J Neurochem. 1991;56:1536–1543. doi: 10.1111/j.1471-4159.1991.tb02048.x. [DOI] [PubMed] [Google Scholar]
- Ding Z-M, Rodd ZA, Engleman EA, McBride WJ. Sensitization of ventral tegmental area dopamine neurons to the stimulating effects of ethanol. Alcohol Clin Exp Res. 2009;33:1571–1581. doi: 10.1111/j.1530-0277.2009.00985.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engleman EA, Keen EJ, Tilford SS, Thielen RJ, Morzorati SL. Ethanol drinking reduces extracellular dopamine levels in the posterior ventral tegmental area of nondependent alcohol-preferring P rats. Alcohol. 2011;45:549–557. doi: 10.1016/j.alcohol.2011.02.304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enrico P, Bouma M, de Vries JB, Westerink BHC. The role of afferents to the ventral tegmental area in the handling stress-induced increase in the release of dopamine in the medial prefrontal cortex: a dual-probe microdialysis study in the rat brain. Brain Res. 1998;779:205–213. doi: 10.1016/s0006-8993(97)01132-3. [DOI] [PubMed] [Google Scholar]
- Ferrani-Kile K, Randall PK, Leslie SW. Acute ethanol affects phosphorylation state of the NMDA receptor complex: implication of tyrosine phosphatases and protein kinase A. Mol Brain Res. 2003;115:78–86. doi: 10.1016/s0169-328x(03)00186-4. [DOI] [PubMed] [Google Scholar]
- Floresco SB, West AR, Ash B, Moore H, Grace AA. Afferent modulation of dopamine neuron firing differentially regulates tonic and phasic dopamine transmission. Nat Neurosci. 2003;6:968–973. doi: 10.1038/nn1103. [DOI] [PubMed] [Google Scholar]
- Franklin KM, Engleman EA, Ingraham CM, McClaren JA, Keith CM, McBride WJ, Murphy JM. A single, moderate ethanol exposure alters extracellular dopamine levels and dopamine D2 receptor function in the nucleus accumbens of Wistar rats. Alcohol Clin Exp Res. 2009;33:1721–1730. doi: 10.1111/j.1530-0277.2009.01009.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao M, Jin Y, Yang K, Zhang D, Lukas RJ, Wu J. Mechanisms involved in systemic nicotine-induced glutamatergic synaptic plasticity on dopamine neurons in the ventral tegmental area. J Neurosci. 2010;30:13814–13825. doi: 10.1523/JNEUROSCI.1943-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gessa GL, Muntoni F, Collu M, Vargiu L, Mereu G. Low doses of ethanol activate dopaminergic neurons in the ventral tegmental area. Brain Res. 1985;348:201–203. doi: 10.1016/0006-8993(85)90381-6. [DOI] [PubMed] [Google Scholar]
- Gonon FG. Nonlinear relationship between impulse flow and dopamine released by rat midbrain dopaminergic neurons as studied by in vivo electrochemistry. Neuroscience. 1988;24:19–28. doi: 10.1016/0306-4522(88)90307-7. [DOI] [PubMed] [Google Scholar]
- Grace A, Bunney B. The control of firing pattern in nigral dopamine neurons: burst firing. J Neurosci. 1984;4:2877–2890. doi: 10.1523/JNEUROSCI.04-11-02877.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grace AA, Bunney BS. Intracellular and extracellular electrophysiology of nigral dopaminergic neurons – 1. Identification and characterization. Neuroscience. 1983;10:301–315. doi: 10.1016/0306-4522(83)90135-5. [DOI] [PubMed] [Google Scholar]
- Grace AA, Onn S-P. Morphology and electrophysiological properties of immunocytochemically identified rat dopamine neurons recorded in vitro. J Neurosci. 1989;9:3463–3481. doi: 10.1523/JNEUROSCI.09-10-03463.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hendricson AW, Maldve RE, Salinas AG, Theile JW, Zhang TA, Diaz LM, Morrisett RA. Aberrant synaptic activation of N-Methyl-D-Aspartate receptors underlies ethanol withdrawal hyperexcitability. J Pharmacol Exp Ther. 2007;321:60–72. doi: 10.1124/jpet.106.111419. [DOI] [PubMed] [Google Scholar]
- Hopf FW, Martin M, Chen BT, Bowers MS, Mohamedi MM, Bonci A. Withdrawal from intermittent ethanol exposure increases probability of burst firing in VTA neurons in vitro. J Neurophysiol. 2007;98:2297–2310. doi: 10.1152/jn.00824.2007. [DOI] [PubMed] [Google Scholar]
- Hubscher C, Brooks D, Johnson J. A quantitative method for assessing stages of the rat estrous cycle. Biotech Histochem. 2005;80:79–87. doi: 10.1080/10520290500138422. [DOI] [PubMed] [Google Scholar]
- Ikemoto S. Dopamine reward circuitry: Two projection systems from the ventral midbrain to the nucleus accumbens–olfactory tubercle complex. Brain Res Rev. 2007;56:27–78. doi: 10.1016/j.brainresrev.2007.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin C, Woodward JJ. Effects of 8 different NR1 splice variants on the ethanol inhibition of recombinant NMDA receptors. Alcohol Clin Exp Res. 2006;30:673–679. doi: 10.1111/j.1530-0277.2006.00079.x. [DOI] [PubMed] [Google Scholar]
- Johnson SW, North RA. Two types of neurons in the rat ventral tegmental area and their synaptic inputs. J Physiol. 1992;450:455–468. doi: 10.1113/jphysiol.1992.sp019136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson S, Seutin V, North R. Burst firing in dopamine neurons induced by N-methyl-D-aspartate: role of electrogenic sodium pump. Science. 1992;258:665–667. doi: 10.1126/science.1329209. [DOI] [PubMed] [Google Scholar]
- Karreman M, Westerink BHC. Excitatory amino acid receptors in the ventral tegmental area regulate dopamine release in the ventral striatum. J Neurochem. 1996;67:601–607. doi: 10.1046/j.1471-4159.1996.67020601.x. [DOI] [PubMed] [Google Scholar]
- Kelland MD, Freeman AS, Chiodo LA. Chloral hydrate anesthesia alters the responsiveness of identified midbrain dopamine neurons to dopamine agonist administration. Synapse. 1989;3:30–37. doi: 10.1002/syn.890030105. [DOI] [PubMed] [Google Scholar]
- Kim SH, Choi YM, Chung S, Uhm DY, Park MK. Two different Ca2+-dependent inhibitory mechanisms of spontaneous firing by glutamate in dopamine neurons. J Neurochem. 2004;91:983–995. doi: 10.1111/j.1471-4159.2004.02783.x. [DOI] [PubMed] [Google Scholar]
- Kwint H-F, de Vries JB. Eating-induced dopamine release from mesolimbic neurons is mediated by NMDA receptors in the ventral tegmental area: A dual-probe microdialysis study. J Neurochem. 1997;69:662–668. doi: 10.1046/j.1471-4159.1997.69020662.x. [DOI] [PubMed] [Google Scholar]
- Lack AK, Diaz MR, Chappell A, DuBois DW, McCool BA. Chronic ethanol and withdrawal differentially modulate pre- and postsynaptic function at glutamatergic synapses in rat basolateral amygdala. J Neurophysiol. 2007;98:3185–3196. doi: 10.1152/jn.00189.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Margolis EB, Lock H, Hjelmstad GO, Fields HL. The ventral tegmental area revisited: is there an electrophysiological marker for dopamine neurons? J Physiol. 2006;577:907–924. doi: 10.1113/jphysiol.2006.117069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Margolis EB, Mitchell JM, Ishikawa J, Hjelmstad GO, Fields HL. Midbrain dopamine neurons: projection target determines action potential duration and dopamine D2 receptor inhibition. J Neurosci. 2008;28:8908–8913. doi: 10.1523/JNEUROSCI.1526-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin D, Morrisett RA, Bian XP, Wilson WA, Swartzwelder HS. Ethanol inhibition of NMDA mediated depolarizations is increased in the presence of Mg2+ Brain Res. 1991;546:227–234. doi: 10.1016/0006-8993(91)91486-k. [DOI] [PubMed] [Google Scholar]
- Miyakawa T, Yagi T, Kitazawa H, Yasuda M, Kawai N, Tsuboi K, Niki H. Fyn-kinase as a determinant of ethanol sensitivity: relation to NMDA receptor function. Science. 1997;278:698–701. doi: 10.1126/science.278.5338.698. [DOI] [PubMed] [Google Scholar]
- Morzorati SL, Marunde RL, Downey D. Limited access to ethanol increases the number of spontaneously active dopamine neurons in the posterior ventral tegmental area of nondependent P rats. Alcohol. 2010;44:257–264. doi: 10.1016/j.alcohol.2010.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morzorati SL, Marunde RL. Comparison of VTA dopamine neuron activity in lines of rats selectively bred to prefer or avoid alcohol. Alcohol Clin Exp Res. 2006;30:991–997. doi: 10.1111/j.1530-0277.2006.00113.x. [DOI] [PubMed] [Google Scholar]
- Murphy JM, Gatto GJ, Waller MB, McBride WJ, Lumeng L, Li TK. Effects of scheduled access on ethanol intake by the alcohol-preferring (P) line of rats. Alcohol. 1986;3:331–336. doi: 10.1016/0741-8329(86)90010-8. [DOI] [PubMed] [Google Scholar]
- Ortiz J, Fitzgerald LW, Charlton M, Lane S, Trevisan L, Guitart X, Shoemaker W, Duman RS, Nestler EJ. Biochemical actions of chronic ethanol exposure in the mesolimbic dopamine system. Synapse. 1995;21:289–298. doi: 10.1002/syn.890210403. [DOI] [PubMed] [Google Scholar]
- Overton P, Clark D. Iontophoretically administered drugs acting at the N-methyl-D-aspartate receptor modulate burst firing in A9 dopamine neurons in the rat. Synapse. 1992;10:131–140. doi: 10.1002/syn.890100208. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. 4th ed. New York: Academic Press; 1998. [Google Scholar]
- Pfaus JG, Damsma G, Wenkstern D, Fibiger HC. Sexual activity increases dopamine transmission in the nucleus accumbens and striatum of female rats. Brain Res. 1995;693:21–30. doi: 10.1016/0006-8993(95)00679-k. [DOI] [PubMed] [Google Scholar]
- Pinheiro JC, Bates DM. Mixed-effects models in S and S-PLUS. New York: Springer-Verlag; 2000. [Google Scholar]
- Rabe CS, Tabakoff B. Glycine site-directed agonists reverse the actions of ethanol at the N-methyl-D-aspartate receptor. Mol Pharmacol. 1990;38:753–757. [PubMed] [Google Scholar]
- Rodd ZA, Melendez RI, Bell RL, Kuc KA, Zhang Y, Murphy JM, McBride WJ. Intracranial self-administration of ethanol within the ventral tegmental area of male Wistar rats: Evidence for involvement of dopamine neurons. J Neurosci. 2004;24:1050–1057. doi: 10.1523/JNEUROSCI.1319-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schultz W. Predictive reward signal of dopamine neurons. J Neurophysiol. 1998;80:1–27. doi: 10.1152/jn.1998.80.1.1. [DOI] [PubMed] [Google Scholar]
- Smith AD, Weiss F. Ethanol exposure differentially alters central monoamine neurotransmission in alcohol-preferring versus -nonpreferring rats. J Pharmacol Exp Ther. 1999;288:1223–1228. [PubMed] [Google Scholar]
- Sombers LA, Beyene M, Carelli RM, Mark Wightman R. Synaptic overflow of dopamine in the nucleus accumbens arises from neuronal activity in the ventral tegmental area. J Neurosci. 2009;29:1735–1742. doi: 10.1523/JNEUROSCI.5562-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stone TW. Microiontophoresis and Pressure Ejection. New York: John Wiley & Sons; 1985. [Google Scholar]
- Stuber GD, Hopf FW, Hahn J, Cho SL, Guillory A, Bonci A. Voluntary ethanol intake enhances excitatory synaptic strength in the ventral tegmental area. Alcohol Clin Exp Res. 2008;32:1714–1720. doi: 10.1111/j.1530-0277.2008.00749.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suaud-Chagny MF, Chergui K, Chouvet G, Gonon F. Relationship between dopamine release in the rat nucleus accumbens and the discharge activity of dopaminergic neurons during local in vivo application of amino acids in the ventral tegmental area. Neuroscience. 1992;49:63–72. doi: 10.1016/0306-4522(92)90076-e. [DOI] [PubMed] [Google Scholar]
- Thielen RJ, Engleman EA, Rodd ZA, Murphy JM, Lumeng L, Li T-K, McBride WJ. Ethanol drinking and deprivation alter dopaminergic and serotonergic function in the nucleus accumbens of alcohol-preferring rats. J Pharmacol Exp Ther. 2004;309:216–225. doi: 10.1124/jpet.103.059790. [DOI] [PubMed] [Google Scholar]
- Thorin C, Mallem MY, Noireaud J, Gogny M, Desfontis J-C. Nonlinear mixed effects models applied to cumulative concentration–response curves. J Pharm Pharmacol. 2010;62:339–345. doi: 10.1211/jpp.62.03.0008. [DOI] [PubMed] [Google Scholar]
- Vonesh EF, Carter RL. Mixed-effects nonlinear regression for unbalanced repeated measures. Biometrics. 1992;48:1–17. [PubMed] [Google Scholar]
- Waller MB, McBride WJ, Lumeng L, Li T-K. Induction of dependence on ethanol by free-choice drinking in alcohol-preferring rats. Pharmacol Biochem Behav. 1982;16:501–507. doi: 10.1016/0091-3057(82)90459-2. [DOI] [PubMed] [Google Scholar]
- Wang T, French ED. Electrophysiological evidence for the existence of NMDA and non-NMDA receptors on rat ventral tegmental dopamine neurons. Synapse. 1993;13:270–277. doi: 10.1002/syn.890130310. [DOI] [PubMed] [Google Scholar]
- Weiss F, Lorang MT, Bloom FE, Koob GF. Oral alcohol self-administration stimulates dopamine release in the rat nucleus accumbens: genetic and motivational determinants. J Pharmacol Exp Ther. 1993;267:250–258. [PubMed] [Google Scholar]
- Westerink B, Kwint H, deVries J. The pharmacology of mesolimbic dopamine neurons: a dual-probe microdialysis study in the ventral tegmental area and nucleus accumbens of the rat brain. J Neurosci. 1996;16:2605–2611. doi: 10.1523/JNEUROSCI.16-08-02605.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Windels F, Kiyatkin EA. General anesthesia as a factor affecting impulse activity and neuronal responses to putative neurotransmitters. Brain Res. 2006;1086:104–116. doi: 10.1016/j.brainres.2006.02.064. [DOI] [PubMed] [Google Scholar]
- Wu M, Hrycyshyn AW, Brudzynski SM. Subpallidal outputs to the nucleus accumbens and ventral tegmental area: anatomical and electrophysiological studies. Brain Res. 1992;740:151–161. doi: 10.1016/s0006-8993(96)00859-1. [DOI] [PubMed] [Google Scholar]
- Xiao C, Shao X, Olive M, Griffin W, III, Li K-Y, Krnjevic K, Zhou C, Ye J-H. Ethanol facilitates glutamatergic transmission to dopamine neurons in the ventral tegmental area. Neuropsychopharmacology. 2008;34:307–318. doi: 10.1038/npp.2008.99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshida M, Yokoo H, Mizoguchi K, Kawahara H, Tsuda A, Nishikawa T, Tanaka M. Eating and drinking cause increased dopamine release in the nucleus accumbens and ventral tegmental area in the rat: Measurement by in vivo microdialysis. Neurosci Lett. 1992;139:73–76. doi: 10.1016/0304-3940(92)90861-z. [DOI] [PubMed] [Google Scholar]
- Yun IA, Wakabayashi KT, Fields HL, Nicola SM. The ventral tegmental area is required for the behavioral and nucleus accumbens neuronal firing responses to incentive cues. J Neurosci. 2004;24:2923–2933. doi: 10.1523/JNEUROSCI.5282-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zellner MR, Kest K, Ranaldi R. NMDA receptor antagonism in the ventral tegmental area impairs acquisition of reward-related learning. Behav Brain Res. 2009;197:442–449. doi: 10.1016/j.bbr.2008.10.013. [DOI] [PubMed] [Google Scholar]
- Zweifel LS, Parker JG, Lobb CJ, Rainwater A, Wall VZ, Fadok JP, Darvas M, Kim MJ, Mizumori SJY, Paladini CA, Phillips PEM, Palmiter RD. Disruption of NMDAR-dependent burst firing by dopamine neurons provides selective assessment of phasic dopamine-dependent behavior. Proc Natl Acad Sci USA. 2009;106:7281–7288. doi: 10.1073/pnas.0813415106. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.