Abstract
Heart disease is the leading cause of death in the United States. Recent studies demonstrate that fetal programming of PKCε gene repression results in ischemia-sensitive phenotype in the heart. The present study tests the hypothesis that increased norepinephrine causes epigenetic repression of PKCε gene in the heart via Nox1-dependent reactive oxygen species (ROS) production. Prolonged norepinephrine treatment increased ROS production in fetal rat hearts and embryonic ventricular myocyte H9c2 cells via a selective increase in Nox1 expression. Norepinephrine-induced ROS resulted in an increase in PKCε promoter methylation at Egr-1 and Sp-1 binding sites, leading to PKCε gene repression. N-acetylcysteine, diphenyleneiodonium, and apocynin blocked norepinephrine-induced ROS production and the promoter methylation, and also restored PKCε mRNA and protein to control levels in vivo in fetal hearts and in vitro in embryonic myocyte cells. Accordingly, norepinephrine-induced ROS production, promoter methylation, and PKCε gene repression were completely abrogated by knockdown of Nox1 in cardiomyocytes. These findings provide evidence of a novel interaction between elevated norepinephrine and epigenetic repression of PKCε gene in the heart mediated by Nox1-dependent oxidative stress and suggest new insights of molecular mechanisms linking the heightened sympathetic activity to aberrant cardioprotection and increased ischemic vulnerability in the heart.—Xiong, F., Xiao, D., Zhang, L. Norepinephrine causes epigenetic repression of PKCε gene in rodent hearts by activating Nox1-dependent reactive oxygen species production.
Keywords: ischemic heart disease, sympathetic activity, oxidative stress, promoter methylation
Heart disease is the leading cause of death in the United States (1). Among other risk factors, recent epidemiological studies have demonstrated a clear association of adverse intrauterine environment with an increased risk of hypertension and ischemic heart disease in adulthood (2–5). Although the mechanisms linking fetal stress and heightened ischemic vulnerability of the heart in later life are poorly understood, studies in several different animal models have demonstrated that fetal programming of protein kinase Cε (PKCε) gene repression in the heart is a congruent cause of increased heart susceptibility to ischemia and reperfusion injury later in life (6–12). Among other mechanisms, PKCε plays a pivotal role of cardioprotection in the setting of heart ischemia and reperfusion injury (13–15). Studies in a PKCε-knockout mouse model have demonstrated that PKCε expression is not required for cardiac function under normal physiological conditions, but PKCε activation is necessary and sufficient for acute cardioprotection during cardiac ischemia and reperfusion (16).
Recently, we demonstrated that a novel mechanism of promoter methylation occurred at non-cytosine-phosphate-guanine (CpG) islands, sequence-specific transcription factor binding sites, in subtle epigenetic modifications of PKCε gene repression in the developing heart in response to fetal stresses, resulting in programming of ischemia-sensitive phenotype of the heart in offspring (11, 12). We further demonstrated that elevated cardiac norepinephrine content in response to fetal stress played a key role in mediating promoter methylation and PKCε gene repression in the heart (12). However, little is known about the mechanisms linking heightened norepinephrine and epigenetic modification of PKCε promoter methylation in the heart. A recent study suggests that increased reactive oxygen species (ROS) may affect DNA methylation patterns that lead to aberrant gene expressions (17). Given that norepinephrine may cause oxidative stress and increase ROS in cardiomyocytes via NADPH oxidases (Noxs; refs. 18, 19), we sought to investigate the role of ROS in norepinephrine-mediated PKCε promoter methylation in the heart. Herein, we present evidence of a novel interaction between elevated norepinephrine and epigenetic repression of PKCε gene in the heart mediated by Nox1-dependent oxidative stress and suggest new insights of molecular mechanisms linking the heightened sympathetic activity to aberrant cardioprotection and increased ischemic vulnerability in the heart.
MATERIALS AND METHODS
Experimental animals
Hearts were isolated from d 17 fetal rats and cultured in M199 (Hyclone, South Logan, UT, USA) supplemented with 10% fetal bovine serum at 37°C in 95% air/5% CO2 for 24 h, followed by 48 h of treatments with norepinephrine and ROS inhibitors, as reported previously (7, 11, 12). To investigate in vivo effects of norepinephrine, pregnant rats were treated with nicotine that increased norepinephrine content in the fetal heart, as previously reported (12). Briefly, time-dated pregnant rats were randomly divided into 3 groups: saline control; nicotine treatment (4 μg/kg/min from d 4 to 21 of gestation); and nicotine treatment plus N-acetylcysteine (NAC) administration in drinking water (500 mg/kg/d), as described previously (12). Hearts were isolated from d 21 fetuses. To isolated fetal hearts, pregnant rats were anesthetized with 75 mg/kg ketamine and 5 mg/kg xylazine injected intramuscularly. The adequacy of anesthesia was determined by the loss of a pedal withdrawal reflex and any other reaction from the animal in response to pinching the toe, tail, or ear of the animal. In addition, even respiration rate of the animal under anesthesia was closely monitored, and an increased respiration rate was used as a sign that anesthesia was too light. After removing fetuses, pregnant rats were killed by removing the hearts. Fetuses were sacrificed by decapitation, and hearts were collected for the studies. All procedures and protocols used in the present study were approved by the Institutional Animal Care and Use Committee of Loma Linda University and followed the guidelines by the U.S. National Institutes of Health Guide for the Care and Use of Laboratory Animals.
Cell culture and drug treatment
Rat embryonic ventricular myocyte H9c2 cells were maintained in Dulbecco's modified Eagle medium (DMEM) supplemented with 10% fetal bovine serum and 1% penicillin/streptomycin, and were cultured at 37°C in 95% air/5% CO2. The experiments were performed at 70 to 80% confluence, as described previously (11, 12). All drugs used were obtained from Sigma-Aldrich (St. Louis, MO, USA). Cells were treated with norepinephrine for 48 h with medium changed at 24 h intervals. The α1-adrenocepter antagonist prazosin was used at concentration of 1 μM. A DNA methylation inhibitor, 5-aza-2′-deoxycytidine, was used at concentrations of 10 μM. The ROS inhibitor NAC was used at concentrations of 1 mM, apocynin at 0.5 mM, and diphenyleneiodonium (DPI) at 10 μM. A mitochondrial respiratory inhibitor, rotenone, was used at concentration of 20 μM.
Western blot
Tissues were homogenized in the RIPA lysis buffer (Pierce, Rockford, IL, USA) supplemented with Halt proteinase inhibitor cocktail (Pierce) and incubated on ice for 30 min to obtain the whole-cell protein extraction. H9c2 cells were directly lysed in RIPA buffer for 30 min on ice to obtain the protein extraction. Nuclear extraction was prepared from hearts and H9c2 cells using NXTRACT CelLytic Nuclear Extraction Kit (Sigma-Aldrich). The protein concentration was determined with BCA protein assay kit (Pierce), following the manufacturer's instructions. Western blot was carried out as described previously (7, 11, 12). Briefly, equal amounts of protein samples were separated with 10% SDS-PAGE and transferred onto nitrocellulose membranes. The membranes were blocked with 5% nonfat milk in TBS for 1 h at room temperature. The membranes were then probed with primary antibodies against PKCε (Santa Cruz Biotechnology, Santa Cruz, CA, USA), specificity protein 1 (Sp1; Active Motif, Carlsbad, CA, USA), β-actin (Sigma-Aldrich), Nox1 (Sigma-Aldrich), Nox2 (BD Biosciences, San Jose, CA, USA) and Nox4 (Abcam, Cambridge, MA, USA) at 4°C overnight. After washing in TBS, membranes were incubated with horseradish peroxidase-conjugated secondary antibodies. Proteins were visualized with enhanced chemiluminescence reagents, and blots were exposed to Hyperfilm (GE Healthcare, Piscataway, NJ, USA). The results were quantified with the Kodak electrophoresis documentation and analysis system and Kodak ID image analysis software (Eastman Kodak, Rochester, NY, USA). The target protein abundance was normalized to the abundance of β-actin.
Real-time RT-PCR
Total RNA was isolated with TRIzol reagent (Invitrogen, Carlsbad, CA, USA) and subjected to reverse transcription with iScript cDNA synthesis kit (Bio-Rad, Hercules, CA, USA), following the manufacturer's instructions. Briefly, 1 μg total RNA was reverse transcribed into cDNA in a 20 μl volume reaction following the protocol of 42°C for 30 min and 85°C for 5 min. The mRNA abundance of target gene was measured with real-time PCR using iQ SYBR Green Supermix (Bio-Rad) as described previously, and amplification of β-actin was used as internal reference (7, 11, 12). Primers used for PKCε were 5′-gcgaagcccctaagacaat-3′ (forward) and 5′-caccccagatgaaatccctac-3′ (reverse). Real-time PCR was performed in a final volume of 25 μl, and each PCR reaction mixture consisted of 600 nM of primers and iQ SYBR Green Supermix containing 0.625 U hot-start Taq polymerase; 400 μM each of dATP, dCTP, dGTP, and dTTP; 100 mM KCl; 16.6 mM ammonium sulfate; 40 mM Tris-HCl; 6 mM MgSO4; SYBR Green I; and 20 nM fluorescing and stabilizers. We used the following real time-PCR protocol: 95°C for 10 min, followed by 40 cycles of 95°C for 15 s, annealing for 20 s at appropriate temperature depending on the primer sequence, 72°C for 10 s. Serial dilutions of the positive control were done on each plate to create a standard curve for the quantification. PCR was done in triplicate, and threshold cycle numbers were averaged for each sample.
Quantitative methylation-specific PCR
Genomic DNA was isolated from fetal hearts or H9c2 cells using a GenElute Mammalian Genomic DNA Mini-Prep kit (Sigma-Aldrich) as described previously (8, 11, 12) and subjected to bisulfite modification with the Gold Methylation kit (Zymo Research, Irvine, CA, USA), following the manufacturer's instructions. Bisulfite-treated DNA was used as template for real-time methylation-specific PCR (MSP) using primers designed to amplify promoter fragments containing possible CpG methylation sites based on our previous sequencing of rat PKCε promoter (8, 11, 12). Real-time MSP was performed using the iQ SYBR Green Supermix with iCycler real-time PCR system (Bio-Rad).
Chromatin immunoprecipitation (ChIP)
ChIP assay was performed using the Chip-IT Express Kit (Active Motif), as described previously (7, 11, 12). Briefly, cells were fixed with 1% formaldehyde to cross-link and maintain the DNA/protein interactions. After the reactions were stopped with glycine, cells were washed with phosphate-buffered saline (PBS). Chromatin extracts were sonicated to produce DNA fragments between 100 and 1000 bp. Antibody against Sp1 (Santa Cruz Biotechnology) was incubated with the chromatin extracts to precipitate the transcription factor/DNA complex. Cross-linking was then reversed using a salt solution, proteins were digested with proteinase K, and the antibody-pulled chromatin extracts were subjected to real-time PCR. Two sets of primers flanking the two SP1 binding sites at −346 and −268 were used: 5′-accatttcctctcgacatgc-3′ (forward) and 5′-agatttcaacccggatcctc-3′ (reverse); 5′-agaggatccgggttgaaatc-3′ (forward) and 5′-ctcacctacctttccgaaaca-3′ (reverse).
ROS measurement
The fluorescent indicator 2′,7′-dichlorofluorescin diacetate (DCF-DA) was used to measure intracellular ROS in H9c2 cells, as described previously (20). DCF-DA enters cells, where it is deesterified and converted to the highly fluorescent 2′,7′-dichlorofluorescin (DCF) on oxidation by the intracellular ROS. Cells were plated into black-wall clear-bottom 96-well plates, grown to 50–60% confluence, and then exposed to appropriate treatment. A time-course measurement of ROS was carried out at 4, 10, 16, 24, 36, and 48 h of norepinephrine treatment. After treatment, cells were washed twice with PBS and incubated with DCF-DA at a concentration of 5 μM for 30 min at 37°C in dark. Fluorescence (Ex495nm/Em528nm) was measured using a Synergy HT Multi-Mode Microplate Reader (Bio-Tek Instruments, Inc., Winooski, VT, USA) and normalized to cell count. Total ROS from fetal heart was measured with Oxiselect In Vitro ROS/RNS Assay kit (Cell Biolabs, San Diego, CA, USA), following the manufacturer's instructions. In addition, dihydroethidium (DHE) fluorescence was used to image the ROS in H9c2 cells and fetal heart slides under confocal microscope (21). Frozen fetal heart was cut into sections at 10-μm thicknesses using a Leica CM 3050S cryostat (Leica Microsystems, Wetzlar, Germany). H9c2 cells and fetal heart slides were incubated with DHE (5 μM) at 37°C for 30 min. Images were obtained by measuring the fluorescence intensity (Ex515nm/Em565–605nm) with the Zeiss LSM710 confocal system (Carl Zeiss, Oberkochen, Germany). To measure mitochondrial ROS in H9c2 cells, cells were loaded with MitoTracker Red CM-H2XRos (Invitrogen) at the concentration of 100 nM for 30 min at 37°C (22, 23). The cells were then washed twice with prewarmed PBS and fixed with freshly prepared 4% formaldehyde in DMEM for 15 min at 37°C. After rinsing several times, cells were incubated with ice-cold acetone for 5 min for permeabilization. Images of MitoTracker Red CM-H2XRos fluorescence (Ex579nm/Em599nm) were acquired with the Zeiss LSM 710 confocal microscope and analyzed using the ZEN software. All experiments were done with minimal exposure to light, and fluorescence was normalized to cell count.
siRNA transfection
The Silencer-Select predesigned siRNAs against rat Nox1 and Nox4 genes were obtained from Invitrogen, and two sets of different siRNA sequences (Si-1 and Si-2) against each gene were used in the experiment to exclude nonspecific effects. Nontargeting siRNAs were used as negative control (NC) for the gene-specific siRNAs. The siRNAs were dissolved in nuclease-free water and transfected into H9c2 cells with the siPORT NeoFX agent (Invitrogen), following the manufacturer's instructions. Briefly, H9c2 cells were trypsinized and diluted in normal growth medium and set aside at 37°C. siPORT NeoFX agent was diluted in Opti-MEM I medium (Invitrogen) and incubated for 10 min at room temperature. siRNAs were diluted in Opti-MEM I medium, and then mixed with the diluted siPORT NeoFX agent. The mixture was incubated 10 min at room temperature, and dispensed with H9c2 cells into 24- or 6-well plates, and incubated for 24 h before changing normal growth medium or starting treatment.
Statistical analysis
Data are expressed as means ± se. Statistical significance (P<0.05) was determined by analysis of variance (ANOVA) followed by Neuman-Keuls post hoc testing or Student's t test, where appropriate.
RESULTS
Norepinephrine increases intracellular ROS production
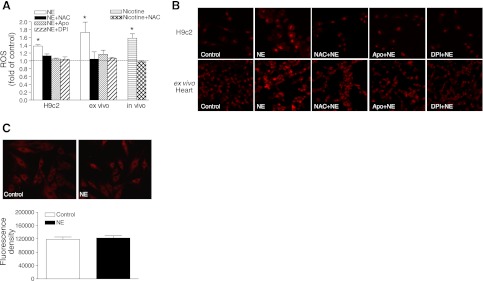
Intracellular ROS was measured by DCF-based quantitative assay kits, as well as by the confocal image of DHE fluorescence, as described in Materials and Methods. Measurement of ROS with the quantitative assay demonstrated that norepinephrine produced a time-dependent increase in intracellular ROS production in H9c2 cells, with the peak at 16 h in a α1-adrenoreceptor-dependent manner (Supplemental Fig. S1). Consistent with the findings in H9c2 cells, quantitative ROS measurement showed that ex vivo treatment of fetal hearts with norepinephrine induced ROS production in the hearts, and norepinephrien-mediated ROS production in both H9c2 cells and fetal hearts was inhibited by either a ROS scavenger, NAC, or Nox inhibitors apocynin or DPI (Fig. 1A). Similar findings were obtained with ROS measured by confocal image of DHE fluorescence. The representative confocal images are shown in Fig. 1B, and the quantification of images is shown in Supplemental Fig. S2. Our previous study demonstrated that maternal nicotine administration resulted in a significant increase in norepinephrine content in fetal rat hearts (12); thus, the in vivo effect of norepinephrine on ROS production in fetal hearts was determined in pregnant rats with maternal nicotine administration. As shown in Fig. 1A, maternal nicotine administration produced a comparable increase in ROS production in fetal hearts as was seen in isolated fetal hearts and H9c2 cells treated with norepinephrine, which was blocked by NAC. On the contrary, the mitochondrial ROS levels were not significantly affected by the norepinephrine treatment (Fig. 1C).
Figure 1.
Norepinephrine (NE) increases the ROS production. H9c2 cells and isolated intact fetal hearts (ex vivo) were treated with 10 μM NE in the absence or presence of NAC (1 mM), apocynin (Apo, 0.5 mM), or DPI (10 μM) for 16 h. Pregnant rats were treated with saline or nicotine via osmotic minipumps from d 4 to 21 of gestation in the absence or presence of NAC (500 mg/kg/d) in the drinking water, and 21 d fetal hearts were isolated for analysis (in vivo). A) ROS measurement with DCF-based quantitative assay kits. B) ROS measurement with confocal image of DHE fluorescence. Representative images are shown; quantification of images is shown in Supplemental Fig. S2. C) Mitochondrial ROS measurement with confocal image of MitoTracker Red CM-H2XRos in H9c2 cells. Data are means ± se, n = 6. *P < 0.05 vs. control.
Inhibition of ROS production reverses norpeinephrine-induced PKCε gene repression
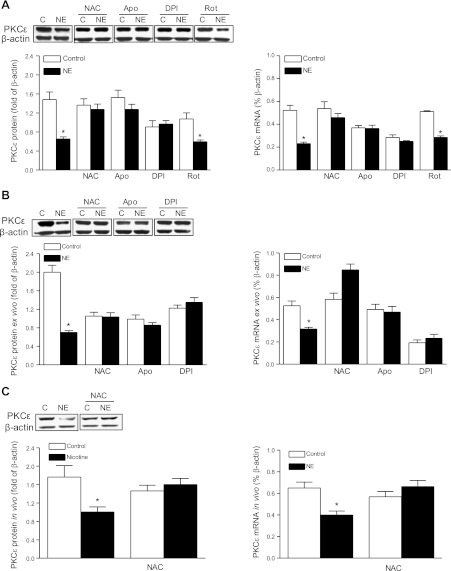
Previously, we demonstrated that norepinephrine inhibited PKCε mRNA and protein expression via α1-adrenoceptors in fetal hearts (12). To determine the cause-and-effect relation of norepinephrine-induced ROS production and PKCε gene repression, fetal hearts and H9c2 cells were treated with norepinephrine in the absence or presence of NAC, apocynin, or DPI. In both fetal hearts and H9c2 cells, norepinephrine-induced down-regulation of PKCε was abrogated by the inhibitors (Fig. 2A, B). Consistent with the lack of effect of norepinephrine on mitochondrial ROS production, a mitochondria complex I inhibitor, rotenone, had no significant effect on norepinephrine-induced down-regulation of PKCε in H9c2 cells (Fig. 2A). To demonstrate the causal role of ROS in norepinephrine-induced PKCε gene repression further in vivo, PKCε mRNA and protein abundance was determined in fetal hearts that were isolated from pregnant rats treated with nicotine in the absence or presence of NAC. As shown in Fig. 2C, maternal nicotine administration produced comparable decreases in PKCε mRNA and protein in fetal hearts as were seen in isolated fetal hearts and H9c2 cells treated with norepinephrine, which was blocked by NAC.
Figure 2.
Inhibition of ROS reverses norepinephrine (NE)-induced PKCε gene repression. H9c2 cells and isolated intact fetal hearts (ex vivo) were treated with 10 μM NE in the absence or presence of NAC (1 mM), apocynin (Apo, 0.5 mM), DPI (10 μM), or rotenone (Rot, 20 μM) for 48 h. Pregnant rats were treated with saline or nicotine via osmotic minipumps from d 4 to 21 of gestation in the absence or presence of NAC (500 mg/kg/d) in the drinking water, and 21 d fetal hearts were isolated for analysis (in vivo). A) H9c2 cells. B) Ex vivo fetal hearts. C) In vivo fetal hearts. Data are means ± se, n = 6. *P < 0.05 vs. control.
Norepinephrine increases CpG methylation of Sp1 binding sites at PKCε promoter
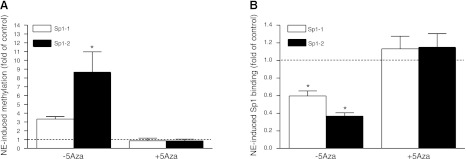
Eight transcription factor binding sites containing CpG dinucleotides at the rat PKCε gene promoter were identified previously, and both the early growth response protein 1 (Egr-1) and two Sp1 binding sites were involved in the PKCε promoter activation (7, 8, 11, 12). Our previous study demonstrated that norepinephrine treatment increased CpG methylation of the Egr-1 binding site at the PKCε promoter in fetal hearts and H9c2 cells (12). We further investigated the effect of norepinephrine on the two Sp1 binding sites at the PKCε promoter. As shown in Fig. 3, treatment of H9c2 cells with norepinephrine caused a significant increase in CpG methylation of Sp1 binding sites at −346 (Sp1-1) and −268 (Sp1-2), resulting in a significant decrease in the binding of Sp1 to the PKCε promoter. DNA methylation inhibitor 5-aza-2′-deoxycytidine abrogated norepinephrine-induced CpG methylation of the Sp1 binding sites and restored the binding of Sp1 to the PKCε promoter (Fig. 3). Norepinephrine treatment had no significant effect on protein abundance of Egr-1 or Sp1 in either cytosol or nuclear extracts (Supplemental Fig. S3).
Figure 3.
5-Aza-2′-deoxycytidine (5-Aza) abrogates norepinephrine (NE)-mediated promoter methylation and restores the Sp1 binding. H9c2 cells were treated with NE for 48 h in the absence or presence of 10 μM 5-Aza. A) CpG methylation of Sp1 binding sites of −346 (Sp1-1) and −268 (Sp1-2) at the PKCε promoter was determined with quantitative methylation-specific PCR. B) Binding of Sp1 to the Sp1 binding sites on the promoter was determined with a ChIP assay. Data are means ± se, n = 4. * P < 0.05 vs. control.
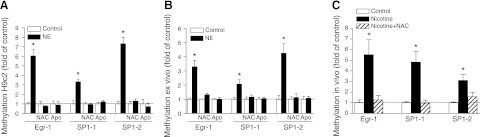
Inhibition of ROS production abrogates norepinephrine-induced CpG methylation of Egr-1 and Sp1 binding sites at PKCε promoter
Previous studies demonstrated that increased CpG methylation of Egr-1 and Sp1 binding sites at the PKCε promoter played an important role in PKCε gene repression (11, 12). To determine further whether inhibition of ROS production restores PKCε gene expression via blocking norepinephrine-induced PKCε promoter methylation, the effect of NAC and apocynin on the methylation status of Egr-1 and Sp1 binding sites at the PKCε gene promoter was examined. As shown in Fig. 4, in H9c2 cells and in fetal hearts of in vivo and ex vivo treatments, NAC or apocynin consistently inhibited norepinephrine-induced CpG methylation of Egr-1 and Sp1 binding sites at the PKCε gene promoter.
Figure 4.
Inhibition of ROS abrogates norepinephrine (NE)-induced CpG methylation of PKCε promoter. H9c2 cells and isolated intact fetal hearts (ex vivo) were treated with 10 μM NE in the absence or presence of NAC (1 mM) and apocynin (Apo, 0.5 mM) for 48 h. Pregnant rats were treated with saline or nicotine via osmotic minipumps from d 4 to 21 of gestation in the absence or presence of NAC (500 mg/kg/d) in the drinking water, and 21 d fetal hearts were isolated for analysis (in vivo). A) H9c2 cells. B) Ex vivo fetal hearts. C) In vivo fetal hearts. CpG methylation of Egr-1 (−1008) and Sp1 (Sp1-1, −346; Sp1-2, −268) binding sites at PKCε promoter was determined with quantitative methylation-specific PCR. Data are means ± se, n = 4–6. * P < 0.05 vs. control.
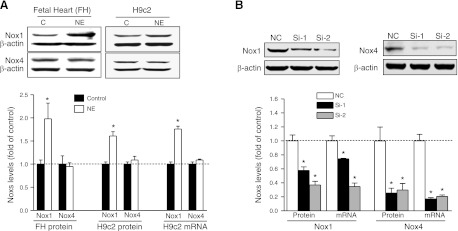
Norepinephrine increases Nox1 expression in fetal rat hearts and H9c2 cells
Nox1, Nox2, and Nox4 are the prevalent isoforms of Nox that expressed in the cardiovascular system. In the present study, we found that both Nox1 and Nox4 were expressed in fetal rat hearts and H9c2 cells (Fig. 5A). In contrast, Nox2 was not detectable in either protein or mRNA levels in fetal rat hearts or in H9c2 cells (Supplemental Fig. S4). Norepinephrine treatment significantly increased Nox1, but not Nox4, mRNA and protein expression levels in both fetal rat hearts and H9c2 cells (Fig. 5A). To determine the specific causal role of Nox1 in norepinephrine-mediated ROS production and PKCε gene repression, we applied the specific siRNAs to H9c2 cells to silence Nox1 and Nox4 expression, respectively. To ensure the specificity of the siRNAs effect, two sets of different sequences for each gene were used. As shown in Fig. 5B, after 24 h of transfection, both of the siRNAs against Nox1 significantly knocked down the expression of Nox1, with a higher efficiency for Nox1 siRNA2. Both siRNA1 and siRNA2 against Nox4 efficiently knocked down the expression levels of Nox4 (Fig. 5B).
Figure 5.
Norepinephrine (NE) increases Nox1 expression. A) Isolated fetal hearts (FH) or H9c2 cells were treated with 10 μM NE for 48 h. B) H9c2 cells were transfected with two sets of different sequences of siRNA (Si-1 and Si-2) against Nox1 or Nox4, respectively. Protein and mRNA abundance of Nox1 and Nox4 was determined. Data are means ± se, n = 6. *P < 0.05 vs. control.
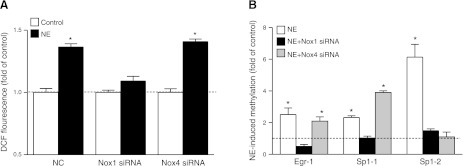
Nox1 knockdown abrogates norepinephrine-induced ROS production and PKCε promoter methylation and restores PKCε gene expression
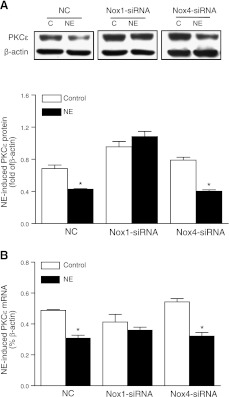
We determined further the cause-and-effect relation of Nox1-mediated ROS production and norepinephrine-induced promoter methylation and PKCε gene repression. As shown in Fig. 6A, knockdown of Nox1, but not Nox4, abolished the norepinephrine-induced ROS production in H9c2 cells. In accordance, Nox1 knockdown completely abrogated norepinephrine-induced CpG methylation of Egr-1 and both Sp1 binding sites at PKCε promoter (Fig. 6B). Nox4 knockdown had no significant effect on norepinephrine-induced methylation of the Egr-1 binding site, increased methylation of the Sp1 (−346) binding site, and inhibited methylation of the Sp1 (−268) binding site, suggesting differential effects of Nox4 on promoter methylation at different binding sites (Fig. 6B). Correspondingly, norepinephrine-induced PKCε gene repression was reversed, and PKCε protein and mRNA expression was restored to the control levels, by knockdown of Nox1, but not Nox4, in H9c2 cells (Fig. 7).
Figure 6.
Nox1 knockdown abrogates norepinephrine (NE)-induced ROS production and PKCε promoter methylation. H9c2 cells were transfected with siRNA against Nox1 or Nox4, respectively, for 24 h, followed by treatment with 10 μM NE for 48 h. A) ROS production was measured with DCF-based quantitative assay kits. B) CpG methylation of Egr-1 (−1008) and Sp1 (Sp1-1, −346; Sp1-2, −268) binding sites at the PKCε promoter, determined with quantitative methylation-specific PCR. Data are means ± se, n = 6. *P < 0.05 vs. control.
Figure 7.
Nox1 knockdown restores PKCε gene expression. H9c2 cells were transfected with siRNA against Nox1 or Nox4, respectively, for 24 h, followed by treatment with 10 μM norepinephrine (NE) for 48 h. A) PKCε protein, measured by Western blot. B) PKCε mRNA, determined by qRT-PCR. Data are means ± se, n = 6. *P < 0.05 vs. control.
DISCUSSION
In the present study, we demonstrate for the first time that norepinephrine-induced ROS production plays an important role in epigenetic modification of PKCε gene promoter methylation patterns in cardiomyocytes in vitro and in vivo, which causes a significant reduction of PKCε protein and mRNA expression. The causal effect of ROS in norepinephrine-induced PKCε gene repression was demonstrated through the use of oxidative stress inhibitors NAC, apocynin, and DPI, which blocked the norpeinephrine-induced methylation and restored PKCε mRNA and protein expression to normal levels. In addition, we demonstrated that the Nox subunit Nox1, but not Nox2 or Nox4, expression was up-regulated by the norepinephrine treatment, and knockdown of Nox1 abrogated norepinephrine-induced ROS production and PKCε gene repression, indicating that Nox1-derived ROS play a pivotal role in norepinephrine-induced epigenetic repression of PKCε gene in the heart.
Consistent with the previous study (11, 12), the norepinephrine-induced increase in PKCε promoter methylation and gene repression observed in fetal hearts in vivo and ex vivo mirrored that found in the embryonic rat ventricular myocyte H9c2 cells in the present study, suggesting congruent underlying mechanisms for each model and demonstrating a comparable model of H9c2 cells in the study of epigenetic regulation of PKCε gene expression patterns in the heart. In the previous studies, we identified 8 transcription factor binding sites on the PKCε promoter, which contain CpG dinucleotides at their core binding sites, and norepinephrine increased CpG methylation of Egr-1 binding site (8, 12). In the present study, we found that norepinephrine treatment also increased CpG methylation at both Sp1 binding sites, resulting in the decreased binding of Sp1 to PKCε promoter. The functional significance of Egr-1 and Sp1 binding sites in regulating PKCε promoter activity has been demonstrated by site-directed deletion and site-specific methylation that significantly reduce the promoter activity in H9c2 cells (7, 8, 12). The finding that nuclear abundance of both Egr-1 and Sp1 was not significantly affected by norepinephrine supports a primary role of promoter methylation in norepinphrine-mediated reduction in transcription factor binding and PKCε gene repression.
In the present study, Nox inhibitors apocynin and DPI completely abrogated norepinephrine-induced ROS production, blocked norepinephrine-mediated PKCε promoter methylation, and restored PKCε expression levels in H9c2 cells and in ex vivo fetal hearts. In contrast, a previous study demonstrated that hypoxia induced epigenetic repression of the PKCε gene via NADPH oxidase-independent ROS productions in the fetal heart (23). The findings that a ROS scavenger NAC reversed both norepinepherine-mediated (the present study) and hypoxia-mediated (23) promoter methylation and PKCε gene repression in fetal cardiomyocytes indicate that it is increased ROS, albeit through different mechanisms, that mediates stress response and PKCε gene repression. Further in vivo studies in the model of maternal nicotine administration with increased norepinephrine content in the fetal heart (12) demonstrated that ROS inhibition with NAC abrogated the heightened PKCε promoter methylation and restored PKCε gene expression in fetal hearts. These findings provide clear evidence of a causal role of ROS in norepinephrine-mediated epigenetic repression of a cardioprotective gene via promoter methylation at non-CpG islands, sequence-specific transcription factor binding sites in the developing heart. Previous studies demonstrated that increased promoter methylation and PKCε gene repression resulted in the heightened heart susceptibility to ischemic injury, which was abrogated by NAC (9, 11, 12, 23). Consistent with the present study, it has been shown that prolonged exposure to ROS causes significant hypermethylation of the E-cadherin promoter in a liver cancer cell line (17). ROS-mediated methylation of E-cadherin promoter involved up-regulation of Snail that recruited epigenetic effectors (i.e., DNA methyltransferase 1) to suppress gene transcription. Interestingly, Snail overexpression alone was sufficient to induce hypermethylation of E-cadherin promoter, suggesting Snail regulation was a key factor in mediating epigenetic modification of gene promoters. Determining whether Snail activity is important in norepinephrine-induced heightened methylation of CpG dinucleotides at transcription factor binding sites of the PKCε promoter deserves further investigation. Furthermore, understanding whether the mechanisms by which norepinephrine via ROS mediates methylations of the Egr-1 and Sp1 binding sites are a broad event (occurring in many genes) or selective (occurring in a few genes) in the heart warrants future investigation.
The causal role of Nox1 in norepinephrine-induced ROS production and PKCε gene repression in cardiomyocytes was further demonstrated with an approach of siRNA to knock down Nox1 or Nox4. Although ROS are produced from different sources, Nox-derived ROS are of particular importance in the heart. Of 7 members of the Nox family identified, Nox1, Nox2, and Nox4 are the main isoforms expressed in the cardiovascular system and contribute to various diseases (24–26). Multiple Nox proteins often exist in one cell, and each Nox plays a specific role according to its specific expression profile, activation stimuli, or intracellular localization. Nox1 has been reported to localize to the plasma membrane in many kinds of cells (27, 28). Unlike Nox1, Nox4 has been reported to mainly localize in several cell compartments, including cytosol, nucleus, mitochondria, and endoplasmic reticulum (29–32). Nox1 expresses at low levels in quiescent cells but can be induced rapidly through an agonist-mediated pathway and exhibit a powerful ability to increase ROS production (33). In contrast, Nox4 expresses in relatively high levels in resting cells and participates in maintaining constitutive low levels of ROS (34).
In the present study, Nox1 and Nox4 protein and mRNA were readily detected in fetal hearts and H9c2 cells, and mRNA abundance of Nox4 was ∼30-fold higher than that of Nox1. Similar ratio of Nox4 to Nox1 abundance was found in vascular smooth muscle cells (35). Unlike Nox1 and Nox4, the Nox2 expression was undetectable at both protein and mRNA levels in both fetal hearts and H9c2 cells. This finding is contrary to a previous study showing the expression of Nox2 in H9c2 cells (36). Whereas the reason for this inconsistency is not clear at present, the positive control in the present study clearly showed the expression of Nox2 in adult rat aorta smooth muscle cells but not in fetal hearts or H9c2 cells. The present finding of lack of Nox2 expression in both fetal hearts and embryonic ventricular myocyte H9c2 cells suggests a novel expression pattern of Nox2 in the developing heart. Whereas the studies of developmental regulation of Nox expression patterns are few, a previous study demonstrated that Nox2 expression in immature cells was constitutively suppressed, and its expression was transcriptionally up-regulated during the maturation of myeloid cells (37).
The present study demonstrated that norepinephrine significantly increased Nox1 mRNA and protein expression in fetal hearts and H9c2 cells, and knockdown of Nox1 abrogated norepinephrine-induced ROS production. In contrast, Nox4 knockdown had no effect on norepinephrine-induced ROS production. In contrast to the previous finding that mitochondria were an important source of hypoxia-induced and Nox-independent ROS production in H9c2 cells (23), the mitochondrial ROS levels were not significantly affected by norepinephrine, demonstrating diverse pathways in regulating intracellular ROS production in myocytes in response to hypoxia and norepinephrine treatment. The lack of mitochondrial contribution to norepinephrine-mediated effect was further demonstrated by the finding that rotenone had no significant effect on norepinephrine-induced down-regulation of PKCε in H9c2 cells. Whereas these findings demonstrate the cause-and-effect relation of Nox1 in norepinephrine-mediated ROS production, additional studies showing that knockdown of Nox1, but not Nox4, blocked norepinephrine-induced CpG methylation of the Egr1 and Sp1 binding sites at PKCε promoter and restored PKCε gene expression, provide further evidence of the causal role of Nox1 in norepinephrine-mediated epigenetic repression of PKCε gene in cardiomyocytes. The finding that Nox4 knockdown had no effect on norepinephrine-induced ROS production but blocked norepinephrine-mediated CpG methylation of the Sp1 binding site at −268 is intriguing, and suggests a ROS-independent effect of Nox4 in regulating promoter methylation. Given that Nox4 resides in the nucleus, these findings suggest a potential novel effect of Nox4 as a prerequisite component of methylation machinery complex targeting at the Sp1−268 binding site. On the contrary, knockdown of Nox4 increased methylation of the Sp1−346 binding site, suggesting a differential mechanism of Nox4 as an inhibitor at different binding sites. These apparent opposite effects of Nox4 on methylation of the two Sp1 binding sites tend to minimize the overall effect of Nox4 on norepinephrine-mediated promoter methylation of PKCε gene. In addition, previous studies with site-directed methylation of PKCε promoter-luciferase constructs selectively at SP1 binding sites at −268 and −346 demonstrated that mutation of CmG at either SP1 site −268 or −346 alone had no significant effect on the promoter activity, but mutation of CmG at the both SP1 binding sites significantly reduced the promoter activity in H9c2 cells (7).
The present investigation provides evidence of a novel interaction between increased norepinephrine and epigenetic repression of PKCε gene in the heart mediated by Nox1-dependent oxidative stress, and suggests new insights of molecular mechanisms linking heightened sympathetic activity to programming of aberrant cardioprotection and increased ischemic vulnerability of the heart. Sympathetic nervous system overactivity plays an important role in the development and progression of cardiovascular diseases (38). A key feature of many cardiovascular diseases is increased sympathetic activity that is organ specific particularly in the heart and kidney rather than a generalized increase. Given that PKCε plays a pivotal role of cardioprotection in the setting of heart ischemia and reperfusion injury and repression of PKCε gene in the heart results in heightened ischemic injury in the heart, the clinical significance of the present findings is 2-fold. First, they provide mechanistic understanding of norepinephrine-mediated oxidative stress as a congruent pathway linking fetal stress and programming of ischemia-sensitive phenotype in the heart and may suggest new leads in the development of preventive diagnosis and therapeutic strategies of fetal programming of heart disease, given that large epidemiological studies clearly indicate a link between fetal stress during gestation and an increased risk of ischemic heart disease later in life (2–5). Second, they also provide novel insights in the understanding of molecular mechanisms underlying sympathetic overactivity-mediated heart vulnerability to ischemic injury in the adult, of which little is known, and may suggest potentially new therapeutic targets in the setting of ischemic heart disease.
Supplementary Material
Acknowledgments
This work was supported by U.S. National Institutes of Health grants HL82779 (L.Z.), HL83966 (L.Z.), HL110125 (L.Z.), and DA032510 (D.X.), and by California Tobacco-Related Disease Research Program Award 18KT-0024 (D.X.).
The authors declare no conflicts of interest.
This article includes supplemental data. Please visit http://www.fasebj.org to obtain this information.
- CHIP
- chromatin immunoprecipitation
- CpG
- cytosine-phosphate-guanine
- DCF
- 2′,7′-dichlorofluorescin
- DCF-DA
- 2′,7′-dichlorofluorescin diacetate
- DHE
- dihydroethidium
- DMEM
- Dulbecco's modified Eagle medium
- DPI
- diphenyleneiodonium
- Egr-1
- early growth response protein 1
- MSP
- methylation-specific PCR
- NAC
- N-acetylcysterine
- NC
- negative control
- Nox
- NADPH oxidase
- PBS
- phosphate-buffered saline
- PKCε
- protein kinase Cε
- ROS
- reactive oxygen species
- Sp1
- specificity protein 1
REFERENCES
- 1. Lloyd-Jones D., Adams R. J., Brown T. M., Carnethon M., Dai S., De Simone G., Ferguson T. B., Ford E., Furie K., Gillespie C., Go A., Greenlund K., Haase N., Hailpern S., Ho P. M., Howard V., Kissela B., Kittner S., Lackland D., Lisabeth L., Marelli A., McDermott M. M., Meigs J., Mozaffarian D., Mussolino M., Nichol G., Roger V. L., Rosamond W., Sacco R., Sorlie P., Thom T., Wasserthiel-Smoller S., Wong N. D., Wylie-Rosett J. (2010) Heart disease and stroke statistics–2010 update: a report from the American Heart Association. Circulation 121, e46–e215 [DOI] [PubMed] [Google Scholar]
- 2. Barker D. J., Osmond C. (1986) Infant mortality, childhood nutrition, and ischaemic heart disease in England and Wales. Lancet 1, 1077–1081 [DOI] [PubMed] [Google Scholar]
- 3. Bateson P., Barker D., Clutton-Brock T., Deb D., D'Udine B., Foley R. A., Gluckman P., Godfrey K., Kirkwood T., Lahr M. M., McNamara J., Metcalfe N. B., Monaghan P., Spencer H. G., Sultan S. E. (2004) Developmental plasticity and human health. Nature 430, 419–421 [DOI] [PubMed] [Google Scholar]
- 4. Gluckman P. D., Hanson M. A., Cooper C., Thornburg K. L. (2008) Effect of in utero and early-life conditions on adult health and disease. N. Engl. J. Med. 359, 61–73 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. McMillen I. C., Robinson J. S. (2005) Developmental origins of the metabolic syndrome: prediction, plasticity, and programming. Physiol. Rev. 85, 571–633 [DOI] [PubMed] [Google Scholar]
- 6. Lawrence J., Xiao D., Xue Q., Rejali M., Yang S., Zhang L. (2008) Prenatal nicotine exposure increases heart susceptibility to ischemia/reperfusion injury in adult offspring. J. Pharmacol. Exp. Ther. 324, 331–341 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Meyer K., Zhang H., Zhang L. (2009) Direct effect of cocaine on epigenetic regulation of PKCepsilon gene repression in the fetal rat heart. J. Mol. Cell. Cardiol. 47, 504–511 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Zhang H., Meyer K. D., Zhang L. (2009) Fetal exposure to cocaine causes programming of Prkce gene repression in the left ventricle of adult rat offspring. Biol. Reprod. 80, 440–448 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Meyer K. D., Zhang H., Zhang L. (2009) Prenatal cocaine exposure abolished ischemic preconditioning-induced protection in adult male rat hearts: role of PKCepsilon. Am. J. Physiol. Heart Circ. Physiol. 296, H1566–H1576 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Xue Q., Zhang L. (2009) Prenatal hypoxia causes a sex-dependent increase in heart susceptibility to ischemia and reperfusion injury in adult male offspring: role of protein kinase C epsilon. J. Pharmacol. Exp. Ther. 330, 624–632 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Patterson A. J., Chen M., Xue Q., Xiao D., Zhang L. (2010) Chronic prenatal hypoxia induces epigenetic programming of PKCε gene repression in rat hearts. Circ. Res. 107, 365–373 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Lawrence J., Chen M., Xiong F., Xiao D., Zhang H., Buchholz J. N., Zhang L. (2011) Foetal nicotine exposure causes PKCepsilon gene repression by promoter methylation in rat hearts. Cardiovasc. Res. 89, 89–97 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Ping P., Zhang J., Pierce W. M., Jr., Bolli R. (2001) Functional proteomic analysis of protein kinase C epsilon signaling complexes in the normal heart and during cardioprotection. Circ. Res. 88, 59–62 [DOI] [PubMed] [Google Scholar]
- 14. Chen L., Hahn H., Wu G., Chen C. H., Liron T., Schechtman D., Cavallaro G., Banci L., Guo Y., Bolli R., Dorn G. W., 2nd, Mochly-Rosen D. (2001) Opposing cardioprotective actions and parallel hypertrophic effects of delta PKC and epsilon PKC. Proc. Natl. Acad. Sci. U. S. A. 98, 11114–11119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Murriel C. L., Mochly-Rosen D. (2003) Opposing roles of delta and epsilonPKC in cardiac ischemia and reperfusion: targeting the apoptotic machinery. Arch. Biochem. Biophys. 420, 246–254 [DOI] [PubMed] [Google Scholar]
- 16. Gray M. O., Zhou H. Z., Schafhalter-Zoppoth I., Zhu P., Mochly-Rosen D., Messing R. O. (2004) Preservation of base-line hemodynamic function and loss of inducible cardioprotection in adult mice lacking protein kinase C epsilon. J. Biol. Chem. 279, 3596–3604 [DOI] [PubMed] [Google Scholar]
- 17. Lim S. O., Gu J. M., Kim M. S., Kim H. S., Park Y. N., Park C. K., Cho J. W., Park Y. M., Jung G. (2008) Epigenetic changes induced by reactive oxygen species in hepatocellular carcinoma: methylation of the E-cadherin promoter. Gastroenterology 135, 2128–2140, 2140 e2121–2128 [DOI] [PubMed] [Google Scholar]
- 18. Liang C., Rounds N. K., Dong E., Stevens S. Y., Shite J., Qin F. (2000) Alterations by norepinephrine of cardiac sympathetic nerve terminal function and myocardial beta-adrenergic receptor sensitivity in the ferret: normalization by antioxidant vitamins. Circulation 102, 96–103 [DOI] [PubMed] [Google Scholar]
- 19. Murdoch C. E., Zhang M., Cave A. C., Shah A. M. (2006) NADPH oxidase-dependent redox signalling in cardiac hypertrophy, remodelling and failure. Cardiovasc. Res. 71, 208–215 [DOI] [PubMed] [Google Scholar]
- 20. Wang H., Joseph J. A. (1999) Quantifying cellular oxidative stress by dichlorofluorescein assay using microplate reader. Free Radic. Biol. Med. 27, 612–616 [DOI] [PubMed] [Google Scholar]
- 21. Csont T., Bereczki E., Bencsik P., Fodor G., Gorbe A., Zvara A., Csonka C., Puskas L. G., Santha M., Ferdinandy P. (2007) Hypercholesterolemia increases myocardial oxidative and nitrosative stress thereby leading to cardiac dysfunction in apoB-100 transgenic mice. Cardiovasc. Res. 76, 100–109 [DOI] [PubMed] [Google Scholar]
- 22. Kuznetsov A. V., Kehrer I., Kozlov A. V., Haller M., Redl H., Hermann M., Grimm M., Troppmair J. (2011) Mitochondrial ROS production under cellular stress: comparison of different detection methods. Anal. Bioanal. Chem. 400, 2383–2390 [DOI] [PubMed] [Google Scholar]
- 23. Patterson A. J., Xiao D., Xiong F., Dixon B., Zhang L. (2012) Hypoxia-derived oxidative stress mediates epigenetic repression of PKCε gene in foetal rat hearts. Cardiovasc. Res. 93, 302–310 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Matsuno K., Yamada H., Iwata K., Jin D., Katsuyama M., Matsuki M., Takai S., Yamanishi K., Miyazaki M., Matsubara H., Yabe-Nishimura C. (2005) Nox1 is involved in angiotensin II-mediated hypertension: a study in Nox1-deficient mice. Circulation 112, 2677–2685 [DOI] [PubMed] [Google Scholar]
- 25. Looi Y. H., Grieve D. J., Siva A., Walker S. J., Anilkumar N., Cave A. C., Marber M., Monaghan M. J., Shah A. M. (2008) Involvement of Nox2 NADPH oxidase in adverse cardiac remodeling after myocardial infarction. Hypertension 51, 319–325 [DOI] [PubMed] [Google Scholar]
- 26. Kuroda J., Ago T., Matsushima S., Zhai P., Schneider M. D., Sadoshima J. (2010) NADPH oxidase 4 (Nox4) is a major source of oxidative stress in the failing heart. Proc. Natl. Acad. Sci. U. S. A. 107, 15565–15570 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Nisimoto Y., Tsubouchi R., Diebold B. A., Qiao S., Ogawa H., Ohara T., Tamura M. (2008) Activation of NADPH oxidase 1 in tumour colon epithelial cells. Biochem. J. 415, 57–65 [DOI] [PubMed] [Google Scholar]
- 28. Shinohara M., Shang W. H., Kubodera M., Harada S., Mitsushita J., Kato M., Miyazaki H., Sumimoto H., Kamata T. (2007) Nox1 redox signaling mediates oncogenic Ras-induced disruption of stress fibers and focal adhesions by down-regulating Rho. J. Biol. Chem. 282, 17640–17648 [DOI] [PubMed] [Google Scholar]
- 29. Ago T., Kuroda J., Pain J., Fu C., Li H., Sadoshima J. (2010) Upregulation of Nox4 by hypertrophic stimuli promotes apoptosis and mitochondrial dysfunction in cardiac myocytes. Circ. Res. 106, 1253–1264 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Block K., Gorin Y., Abboud H. E. (2009) Subcellular localization of Nox4 and regulation in diabetes. Proc. Natl. Acad. Sci. U. S. A. 106, 14385–14390 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Kuroda J., Nakagawa K., Yamasaki T., Nakamura K., Takeya R., Kuribayashi F., Imajoh-Ohmi S., Igarashi K., Shibata Y., Sueishi K., Sumimoto H. (2005) The superoxide-producing NAD(P)H oxidase Nox4 in the nucleus of human vascular endothelial cells. Genes Cells 10, 1139–1151 [DOI] [PubMed] [Google Scholar]
- 32. Hilenski L. L., Clempus R. E., Quinn M. T., Lambeth J. D., Griendling K. K. (2004) Distinct subcellular localizations of Nox1 and Nox4 in vascular smooth muscle cells. Arterioscler. Thromb. Vasc. Biol. 24, 677–683 [DOI] [PubMed] [Google Scholar]
- 33. Lassegue B., Sorescu D., Szocs K., Yin Q., Akers M., Zhang Y., Grant S. L., Lambeth J. D., Griendling K. K. (2001) Novel gp91(phox) homologues in vascular smooth muscle cells: nox1 mediates angiotensin II-induced superoxide formation and redox-sensitive signaling pathways. Circ. Res. 88, 888–894 [DOI] [PubMed] [Google Scholar]
- 34. Van Buul J. D., Fernandez-Borja M., Anthony E. C., Hordijk P. L. (2005) Expression and localization of NOX2 and NOX4 in primary human endothelial cells. Antioxid. Redox Signal. 7, 308–317 [DOI] [PubMed] [Google Scholar]
- 35. Ago T., Kitazono T., Ooboshi H., Iyama T., Han Y. H., Takada J., Wakisaka M., Ibayashi S., Utsumi H., Iida M. (2004) Nox4 as the major catalytic component of an endothelial NAD(P)H oxidase. Circulation 109, 227–233 [DOI] [PubMed] [Google Scholar]
- 36. Borchi E., Parri M., Papucci L., Becatti M., Nassi N., Nassi P., Nediani C. (2009) Role of NADPH oxidase in H9c2 cardiac muscle cells exposed to simulated ischaemia-reperfusion. J. Cell. Mol. Med. 13, 2724–2735 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Skalnik D. G., Strauss E. C., Orkin S. H. (1991) CCAAT displacement protein as a repressor of the myelomonocytic-specific gp91-phox gene promoter. J. Biol. Chem. 266, 16736–16744 [PubMed] [Google Scholar]
- 38. Malpas S. C. (2010) Sympathetic nervous system overactivity and its role in the development of cardiovascular disease. Physiol. Rev. 90, 513–557 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.