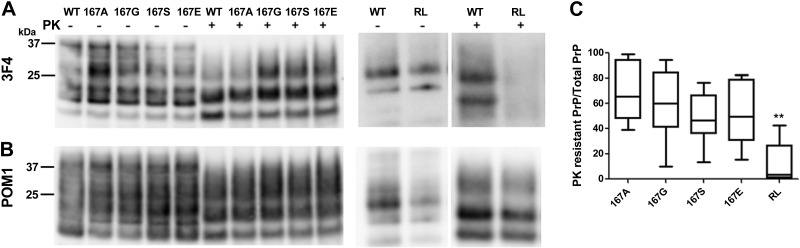
Figure 4.
RML mouse prions in immortalized CAD cells efficiently convert mouse PrPC with amino acid substitutions at position 167. A) Western blot shows that the 3F4 epitope-tagged PrPSc from WT and 167 mutants (167A, 167G, 167S, and 167E) are efficiently converted by the RML prions, whereas the RL mutant with the 170N, 174T substitutions is poorly converted. B) Membrane from panel A immunoblotted with anti-PrP antibody POM1 shows that the endogenous PrPSc levels are unaffected by the PrPSc mutants. C) Ratio of PK-digested PrPSc to total PrP signal relative to WT (normalized to an upper limit of 1.0). There was no significant difference (P<0.01) between WT PrP and the 167 PrP mutants when the raw values were compared. There was a significant difference between the WT and RL mutant (170N, 174T) in a Student's t test. **P < 0.001.