Table 1.
In Situ Intramolecular Schmidt Reaction of Known Substrates[a]
| Entry | Halide | Product | Yield (%) |
Recovery (%) (Azide/Halide) |
||
|---|---|---|---|---|---|---|
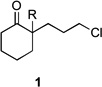 |
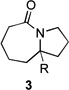 |
|||||
| 1 | 1a | R = H | 3a | R = H | 69 | – |
| 2 | 1a | R = H | 3a | R = H | 76[b] | – |
| 3 | 1b | R = CO2Et | 3b | R = CO2Et | 68 | 5/6 |
| 4 | 1c | R = Ph | 3c | R = Ph | 5 | 60/15 |
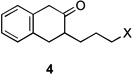 |
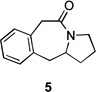 |
|||||
| 5 | 4a | X = Cl | 5 | 60 | –/23 | |
| 6 | 4b | X = Br | 5 | 61 | – | |
 |
 |
|||||
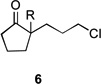
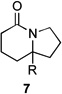
| 7 | 6a | R = H | 7a | R = H | 8 | 37/19 |
| 8 | 6b | R = CO2Et | 7b | R = CO2Et | – | 66/16 |
 |
 |
|||||
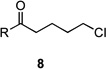
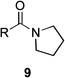
| 9 | 8a | R = Me | 9a | R = Me | 25 | 36/14 |
| 10 | 8b | R = CH2CO2Et | 9b | R = CH2CO2Et | – | 44/7 |
Conditions: 1. Halide 2M in CH3CN, NBu4N3 (1.1 equiv.), 125 W MWI, 50 µL/min. 2. TFA (3 mL, excess) 50 µL/min, 40 PSI back pressure.
Second step of the sequence performed outside of the microwave cavity.
