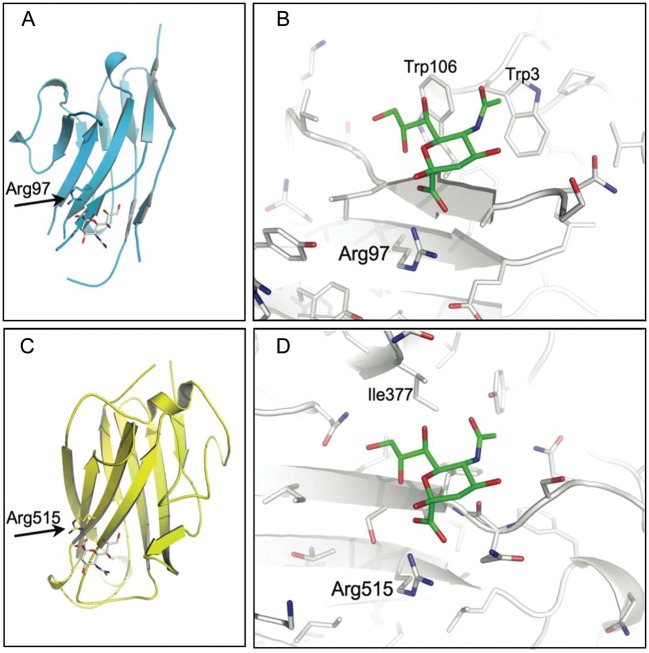
Fig. 1.
Structural alignment of Siglec-1 and CAV-2 fiber knob. The structural alignment based on the ring atoms of the sialic acid is shown for the entire glycan binding site of the V-set domain of Siglec-1 (A, C) and CAV-2 fiber knob protein (B, D). The essential arginine is highlighted, and the sandwich fold of two antiparallel beta-sheets is shown for both structures. The extension of the beta-sheet by the sialic acid is also apparent for both structures. In contrast, the van der Waals interaction of the C9 methylene of the glycerol side chain to Trp-106 in Siglec-1 is not observed for the CAV-2 structure. Moreover, while the N-acteyl group recognition site in Siglec-1 is mediated by hydrophobic contacts originating from Trp-3, other hydrophobic side chains in CAV-2 replace this feature. Hydrogens were omitted for simplification (Siglec-1, pdb id: 1QFO; CAV-2, pdb id: 2WBV).