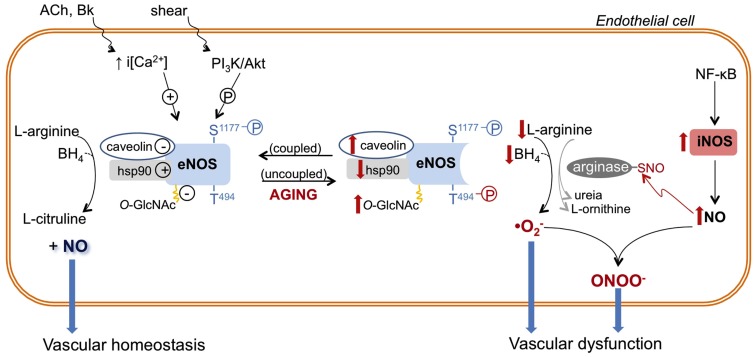
Figure 1.
Role of nitric oxide synthase (NOS) enzymes in endothelial function and aging-associated endothelial dysfunction. Endothelial (e)-NOS isoform converts l-arginine to nitric oxide (NO). eNOS activity is determined by intracellular calcium concentration [i(Ca2+)] and/or its phosphorylation (P) state at different sites (e.g., eNOS is positively and negatively modulated by phosphorylation of serine 1177 (S1177) and threonine 494 (T494), respectively). In addition, eNOS activity is inhibited by its interaction with caveolin-1 and by O-GlcNAc modification, while eNOS association with heat shock protein 90 (hsp90) favors its activation. In endothelial aging, NO synthesis is compromised because eNOS activity is decreased due to increased expression and interaction with caveolin, reduced expression and association with hsp90, reduced phosphorylation of S1177 and increased phosphorylation of T494. In addition, reduced availability of l-arginine and tetrahydrobiopterin (BH4) induces eNOS uncoupling or changes the enzyme to a state that favors superoxide anion generation. In aged endothelial cells, up-regulated iNOS, which may be associated with NF-κB-induced vascular inflammation, produces high levels of NO. NO reacts with cysteine residues of proteins (arginase in this example), forming S-nitrosothiol (-SNO)–arginase, which increases arginase activity and l-arginine consumption. NO directly reacts with produced by uncoupled eNOS, and other vascular sources, generating the harmful reactive nitrogen specie peroxynitrite (ONOO−), which contributes to vascular dysfunction. The aging–associated NOS alterations are depicted in red. ACh, acetylcholine; Bk, bradykinin; PI3K, phosphoinositide-3-kinase.