Abstract
A crucial step in recent theories of human origins is the emergence of strong pair-bonding between males and females accompanied by a dramatic reduction in the male-to-male conflict over mating and an increased investment in offspring. How such a transition from promiscuity to pair-bonding could be achieved is puzzling. Many species would, indeed, be much better off evolutionarily if the effort spent on male competition over mating was redirected to increasing female fertility or survivorship of offspring. Males, however, are locked in a “social dilemma,” where shifting one’s effort from “appropriation” to “production” would give an advantage to free-riding competitors and therefore, should not happen. Here, I first consider simple models for four prominent scenarios of the human transition to pair-bonding: communal care, mate guarding, food for mating, and mate provisioning. I show that the transition is not feasible under biologically relevant conditions in any of these models. Then, I show that the transition can happen if one accounts for male heterogeneity, assortative pair formation, and evolution of female choice and faithfulness. This process is started when low-ranked males begin using an alternative strategy of female provisioning. At the end, except for the top-ranked individuals, males invest exclusively in provisioning females who have evolved very high fidelity to their mates. My results point to the crucial importance of female choice and emphasize the need for incorporating between-individual variation in theoretical and empirical studies of social dilemmas and behaviors.
Keywords: food-for-mating, self-domestication
There are many characteristics that make us a “uniquely unique” species, including those related to morphology, ecology, development, and life history as well as sexual, social, cognitive, linguistic, and cultural traits and abilities (1–4). Both ultimate and proximate mechanisms that were driving their emergence and evolution in hominins are the subject of intensive research efforts and numerous controversies. Recent influential theories link the appearance of some of the unique human features to a major transition in life history strategy that transformed the social structure of early hominins from promiscuous groups to multimale/multifemale groups with strong pair-bonding (4–9). After the new mating system had evolved, a number of subsequent evolutionary transitions became possible. In particular, pair-bonding served as a preadaptation to parental partnership based on the division of labor, which was necessary to offset the disproportionally high costs of raising human children (because of their large brain and delayed maturity). Pair-bonding allowed children to recognize their fathers (and vice versa) on a reliable basis, and subsequently, it led to the emergence of a new type of family that integrated three generations of individuals of both sexes. Recognition of kinship networks simplified the evolution of within-group cooperative behavior, including alloparental care. It also allowed for between-group alliances taking advantage of the bonds between females transferring to other groups and their fathers and brothers remaining in the natal groups.
How such a transition from promiscuity to pair bonding could be achieved is puzzling (4–6, 10–13). The classical explanation of monogamy in primates—that females’ dispersion across a landscape forces males to associate with individual females (14)—does not work for group-living species with strong within-group dominance hierarchies and high-ranked males largely monopolizing mating (15–18). Also problematic are the suggestions that monogamy was a preferred strategy for reducing the risk of infanticide by strange males (19) and that it emerged because male parental care was indispensable to female reproduction. [Data suggest that paternal care had often evolved after monogamy was already established (20).] Recent discussions, instead, focus on communal breeding (4, 6), mate guarding (6), and food-for-mating transactions (5).
However, an important component is missing from these discussions. Many species would, indeed, be much better off evolutionarily if the effort spent on male competition over mating was redirected to increasing female fertility or survivorship of offspring. However, the fact that there is a higher fitness solution to a particular social (or evolutionary) situation does not imply that this solution will be realized. Realizing such a solution may require crossing fitness valleys and/or finding a way to make it stable to the invasion of various mutants. In the context of the transition to pair-bonding, it has been argued that males are locked in a “social dilemma,” where shifting one’s effort from “appropriation” (i.e., contending with other males for mating success) to “production” (i.e., caring and provisioning) would give an advantage to free-riding competitors and therefore, should not happen (10, 21, 22). In fact, a major challenge for the evolutionary theory is to explain the emergence of group-beneficial behaviors and traits that would be resistant to the invasion of cheaters and free riders (23–25).
Here, I first use simple mathematical models to illustrate the power of the social dilemma faced by males, which results in selective forces strongly opposing the shift from appropriation to production. Then, I propose a general scenario extending and making more specific some of the earlier ideas on how to resolve this dilemma.
Results
I consider a population in which individuals interact in groups comprised of N males and N females. Each male divides his effort between two activities potentially increasing his fitness. One activity is contending for status and dominance with other males in the group. The other activity is directed to females and offspring (e.g., caring for offspring and provisioning or guarding females). I posit that the share Si of paternity won by male i in direct competition with other males is given by the standard Tullock contest success function of (Eq. 1)
 |
which is extensively used in economics (22) and evolutionary biology (10, 13, 26, 27). Here, 0 ≤ mi ≤ 1 is the fighting effort of male i, and parameter β > 0 measures the decisiveness of the differences in male fighting effort in controlling the outcome of the competition. Describing the situations where only a few males get most of the matings (which happens in chimpanzees and other species living in hierarchically organized groups) (28, 29) (SI Appendix) requires one to assume that β is sufficiently larger than one (e.g., two to four). Using the information in ref. 10, I assume that female fertility is defined by the function (Eq. 2)
where 0 ≤ y ≤ 1 is a male’s effort to caring or provisioning and α > 0 measures the efficiency of males’ effort. Parameter C can be interpreted as a female’s contribution to her fertility, and it is set to one. If α < 1, the males’ effort is less efficient than the effort of females; if α > 1, there is a synergy between female and male efforts (i.e., the total effect is greater than the sum of the two). It is reasonable to assume that α does not exceed one by too much (10) (SI Appendix).
In the communal care model (10), each male allocates a fraction ci of his effort to caring for offspring (ci + mi = 1). The male care is distributed randomly among all offspring in the group and increases female fertility by a factor 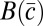 , where
, where 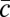 is the average care in the group. In a group with N females, male i wins a share Si of paternity in competition with other males, and his fitness is
is the average care in the group. In a group with N females, male i wins a share Si of paternity in competition with other males, and his fitness is 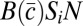 .
.
In the mate-guarding model, male i devotes effort gi to guarding a particular female (gi + mi = 1). Guarding effort gi gives a paternity γgi of the guarded female’s offspring, where 0 ≤ γ ≤ 1 is the guarding efficiency. The total unguarded paternity is 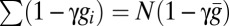 , where
, where 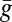 is the average guarding effort. Male i wins share Si of this paternity in competition, and his fitness is defined in the second row of Table 1.
is the average guarding effort. Male i wins share Si of this paternity in competition, and his fitness is defined in the second row of Table 1.
Table 1.
Summary of models
| Model | Variables | Male fitness | Evolution to mi = 1 if |
| Communal care (10) | mi, ci | 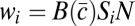 |
α < β(N − 1) |
| Mate guarding | mi, gi | 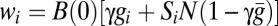 |
γ < β |
| Food for mating | mi, pi | 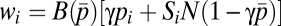 |
α < (β − γ)(N − 1) |
| Mate provisioning | mi, pi | 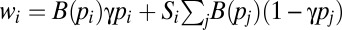 |
α < (β − γ)(N − 1) |
| Pair bonding | mi, pi | 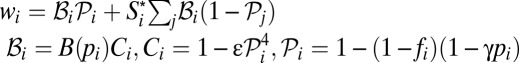 |
SI Appendix |
In the food-for-mating model, each male allocates effort pi to provisioning randomly chosen females (pi + mi = 1). Provisioning at level pi buys paternity in the amount γpi, where 0 ≤ γ ≤ 1 is the efficiency of provisioning to paternity conversion. Provisioning also increases female fertility by a factor 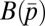 , where
, where 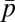 is the average provisioning effort in the group. The total paternity assigned in competition is
is the average provisioning effort in the group. The total paternity assigned in competition is 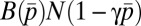 . The male’s fitness is defined in the third row of Table 1, where the first term in the brackets gives paternity bought with food, whereas the second term is paternity won in competition.
. The male’s fitness is defined in the third row of Table 1, where the first term in the brackets gives paternity bought with food, whereas the second term is paternity won in competition.
The mate-provisioning model is similar to the food-for-mating model, except that each male can provision only one female and each female can be provisioned by only one male. As a result of provisioning, the female’s fertility is increased by a factor B(pi). The total paternity assigned in competition is 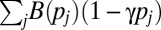 . The male’s fitness is defined in the fourth row of Table 1, where the first and second terms in the brackets give paternity of the provisioned female offspring and paternity won in competition, respectively.
. The male’s fitness is defined in the fourth row of Table 1, where the first and second terms in the brackets give paternity of the provisioned female offspring and paternity won in competition, respectively.
These models can be analyzed using a standard invasion analysis (30–32). A common feature of the four models is that, under the biologically most relevant conditions (i.e., small α, relatively large β ∼ 2–4, and large N ∼ 10) (33, 34), they all predict (Table 1) evolution to a state where all male effort is devoted to fighting (mi = 1). An alternative dynamic, which is the only other possibility in the first three models, is the evolution to a state where all males exhibit an intermediate fighting effort and the rest of their effort goes to caring or provisioning. In the mate-provisioning model, there is also a possibility that the system evolves to a polymorphic state at which a minority of males devotes all effort to provisioning, whereas remaining males devote all their effort to fighting. Such a polymorphic state is analogous to the states observed in producer–scrounger models (35–38); in the present context, “scroungers” are males who do not invest in females but rather, “steal” paternity. However, all these alternatives require α to be large and/or β and N to be small, which seems to be unrealistic under the conditions inferred for hominins. [For example, with β = 3 and N = 10, α would have to be >27 in the communal care model and >18 in the food-for-mating and mate-provisioning models, where in the latter case, I optimistically assumed γ = 1. With such large α-values, the effect of males on female fertility, defined in the model as (1 + p)α, would have to be enormously large.]
The results summarized in Table 1 assume that groups are formed randomly, which implies low probability of genetic relatedness between individuals. In chimpanzees and likely, hominins, within-group genetic relatedness can be somewhat elevated, because only one sex (females) disperses (39–41). The kin selection theory (23, 25, 42) predicts reduced competition in kin groups. Elevated relatedness does, indeed, reduce between-male competition in the communal care, food-for-mating, and mate-provisioning models. (In the mate-guarding model, relatedness has no effect.) However, in realistic situations, the conditions given in Table 1 will not change substantially (SI Appendix).
At the state with m = 1, female fertility [B(0) = 1] is significantly smaller than the fertility that could be achieved [B(1) = 2α] if all males were to devote all their effort to female provisioning or caring for offspring. Males are forced to invest in appropriation rather than production by the logic of social interactions in a promiscuous group, where investing more in offspring means that there is more paternity for other males to steal (10). Thus, the male’s dilemma drives the evolution to a low fitness (payoff) state, which is a feature shared by other social dilemmas (e.g., the Prisoner’s dilemma or the public goods dilemma) (23–25).
These results have implications for some recent theories of human origins. In particular, the works in refs. 4, 6, and 8 argue for the importance of communal breeding during the origin of humans. Ref. 6 also argues for the importance of mate guarding as a preferable strategy after the inequality in strengths between males was reduced by the invention of weapons. My modeling results contradict these arguments. Although switching to communal breeding and mate guarding could increase fitness, it does not happen, because selection is not able to overcome the accompanying free-rider problem.
In contrast, the scenarios, including mate provisioning (5), seem promising because of the “double benefit” of provisioning to males. Indeed, a provisioning male not only gets mating (43), but provisioning also increases fertility of his mate and thus, the number of the male’s offspring. However, some additional factors need to be taken in consideration. Here, I focus on two factors that, arguably, are rather general.
The first factor is inequality between males in their fighting abilities, which is always present (28, 29) because of various genetic, developmental, and environmental factors. With strong inequality in strengths, weaker males do not have as much chance of winning between-male competition, and thus, they may be eager to use alternative reproductive strategies (36, 44, 45). In the model, I will assume that each male is characterized by a constant strength si drawn randomly from a uniform distribution on interval 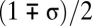 , where parameter 0 ≤ σ ≤ 1 measures the extent of the variation in male strengths.
, where parameter 0 ≤ σ ≤ 1 measures the extent of the variation in male strengths.
As in the mate-provisioning model, let males divide their effort between provisioning a female and contending with other males for mating with multiple females. I will assume that the male fighting effort is conditioned on his dominance rank in the group. This assumption requires one to model a male strategy not as a scalar as above but as a vector 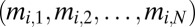 , where mi, j is the fighting effort of a male with genotype i when at rank j. With nonequal males, I postulate that the share of paternity won by male i is given by Tullock function with mi substituted by
, where mi, j is the fighting effort of a male with genotype i when at rank j. With nonequal males, I postulate that the share of paternity won by male i is given by Tullock function with mi substituted by 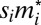 , where
, where 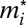 is a rank-dependent fighting effort of male i (Eq. 3):
is a rank-dependent fighting effort of male i (Eq. 3):
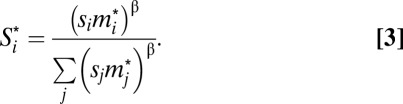 |
The second set of factors is related to the role of females. In the models described so far, females played a passive role. However, because they receive direct benefits from provisioning males, females should be choosy, and they may become, to some extent, faithful to them. [This argument implies that females exert some control over their mating behavior. This assumption is reasonable, because the strongest male(s) can never be in complete control of female mating behavior if there are multiple adult males in the group.] To model these effects, I allow females to differ with respect to their faithfulness. I postulate that the share of paternity obtained by a male with provisioning trait pi from a female with faithfulness fi (0 ≤ fi ≤ 1) is (Eq. 4)
If fi = 0, then 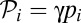 as in the mate-provisioning model. Female faithfulness, if present, is expected to make switching to pair-bonding easier. Indeed, in the mate-provisioning model, if all females have identical faithfulness f, the right-hand side of the inequality in Table 1 must be multiplied by factor
as in the mate-provisioning model. Female faithfulness, if present, is expected to make switching to pair-bonding easier. Indeed, in the mate-provisioning model, if all females have identical faithfulness f, the right-hand side of the inequality in Table 1 must be multiplied by factor 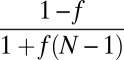 . High faithfulness f, thus, can significantly weaken the conditions for escaping the m = 1 state.
. High faithfulness f, thus, can significantly weaken the conditions for escaping the m = 1 state.
However, polyandry can have multiple genetic and material benefits (including access to better genes, increasing the probability of fertilization, preventing infanticide, receiving support from males in agonistic interactions, etc.) (46–48), and therefore, switching to monogamy can result in fitness costs. Therefore, I conservatively posit that female fertility declines with the paternity 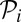 obtained by her pair mate. Specifically, I assume that fertility is reduced by a factor (Eq. 5)
obtained by her pair mate. Specifically, I assume that fertility is reduced by a factor (Eq. 5)
where ε is the maximum reduction of fertility (observed when monogamy is strict; i.e., 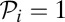 ). This quartic function captures a reasonable assumption that fertility costs of pair-bonding become significant only when paternity
). This quartic function captures a reasonable assumption that fertility costs of pair-bonding become significant only when paternity 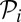 obtained by the partner is sufficiently large.
obtained by the partner is sufficiently large.
Females have an incentive to bond with provisioning males. Simultaneously, provisioning males have an incentive to bond with females who remain faithful to them; the more the male’s effort to provisioning, the stronger the incentive. These factors are expected to lead to nonrandom pair formation. To describe it, I use a simple model in which the probability of a mating bond between male i and female j is proportional to (Eq. 6)
Parameter ω scales the range of possible ψ values: the minimum is one (at p = 0 or f = 0), and the maximum is exp(ω) (at p = f = 1). In the terminology of nonrandom mating models (49, 50), Eq. 6 describes open-ended preference.
As shown above, with high female faithfulness, males are expected to switch to provisioning. Will female faithfulness increase if starting with low values? To get intuition, we can evaluate evolutionary forces acting on female faithfulness. Assuming that the average value of p as well as the variances of f and p are small, the invasion analysis shows (see SI Appendix) that faithfulness f will increase when small if 4γ3p2ε < ω (that is, if the benefit of promiscuity, ε, is not too large, and the assortativeness in pair formation, ω, is strong enough). Increasing female faithfulness f should cause the evolution of increased male provisioning p. As male provisioning trait p increases, female faithfulness f will evolve to higher and higher values until it stabilizes at a level controlled by a balance between selection for good genes and access to food provisioned by males.
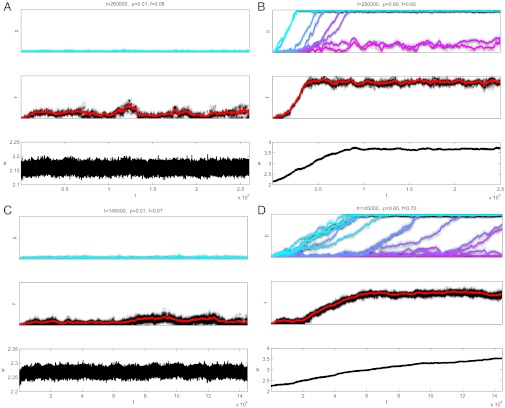
This argument is based on the consideration of separate components of the pair-bonding model. To check this logic, I have performed stochastic individual-based simulations. I considered a finite population of sexual diploid individuals subdivided into G groups. The results confirm the expectation (SI Appendix). When the fitness benefit of promiscuity (ε) is large enough and/or the variation in male strengths (σ) is low, the population evolves to a low fitness state with m = 1 and no female provisioning (Fig. 1 A and C). When both the fitness benefit of promiscuity is not too large and the variation in male strengths is significant, the population exhibits a strikingly different dynamics shifting to a high fitness pair-bonding state with high levels of male provisioning and female faithfulness (Fig. 1 B and D). At the end, except for a very small proportion of the top-ranked individuals, males invest exclusively in provisioning females who have evolved very high fidelity to their mates. The shift to pair-bonding occurs in a sequence of transitions in the strategies of males of different rank, starting from the lowest rank and going up to the highest ranks. Occasionally the model exhibits cycling behavior when both female faithfulness f and male provisioning traits p for the top-ranked males fluctuate (SI Appendix). The cycling occurs because once most males are provisioning, female faithfulness is not selected for anymore. Then selection against monogamy takes over with females evolving decreasing faithfulness, which in turn forces top-ranked males to reduce their provisioning and increase their investment in competition.
Fig. 1.
Examples of long-term evolutionary dynamics. (A and C) Small variation among males (σ = 0.25). (B and D) Large variation among males (σ = 1). (A and B) N = 8, ε = 0.05. (C and D) N = 16, ε = 0.1. Other parameters: α = 1, β = 3, γ = 0.5, ω = 1. In A–D, Top shows male provisioning traits pi for males of different rank from low (cyan) to high (magenta), Middle shows female faithfulness trait f, and Bottom shows the average fitness. The colored curves showing mean trait values are superimposed on the graphs to show the distributions of the traits using the gray color scheme. The numbers on top of sets of graphs show the final generation and the average values of p and f at this generation.
Discussion
The transition from promiscuity to pair-bonding in a species living in hierarchically organized groups requires a mechanism that would resolve the male’s dilemma, i.e., the conflict between investing in appropriation and production, in favor of the latter (10). Several such mechanisms have been recently advanced in the literature, including those focusing on communal care, mate guarding, and mate provisioning (4–8). Using a series of simple models that build on earlier work (10), I have shown that, under biologically realistic conditions (e.g., when the group size is not too small, competition between males is strong, and the effects of male provisioning and care are not too large), the population is not able to escape the low fitness state at which males invest exclusively into competition for mating. This conclusion is not changed qualitatively, even if one accounts for an elevated genetic relatedness between males arising from their philopatry. Note that communal care provided by females, the importance of which has been stressed in a number of recent publications (4, 6–8), is even less likely to become established because of low relatedness between females who disperse to different groups on maturity. Moreover, females may benefit from multiple matings (46–48), which implies additional selection against pair-bonding. The power and implications of the male’s dilemma discussed above have not been generally acknowledged in the discussions of human transition to pair-bonding (10).
The solution of the male’s dilemma proposed here builds on the idea of mate provisioning augmented by the explicit consideration of (i) females’ evolutionary response to provisioning and (ii) the role of males’ dominance ranks in determining their preferred actions. Mate provisioning has double benefits, one of which (mating) is immediate and another (increased fertility and decreased between-birth interval) is delayed. These benefits are most pronounced for low-ranked males who have a low chance of winning a mate in competition with top-ranked males. One, therefore, should expect that it is low-ranked males who will attempt to buy mating by provisioning. Note that, if there are more males at the bottom than at the top of the hierarchy, selection benefiting the “masses” may become stronger than selection benefiting the “elite”. Top-ranked males can easily beat out or chase away the low-ranked males and steal the paternity, making the investment of low-ranked males in production wasteful. However, after females start developing preferences for being provisioned, the low-ranked males’ investments start to pay off. In the model presented here, male provisioning and female faithfulness coevolve in a self-reinforcing manner. At the end, except for a very small proportion of the top-ranked individuals, males invest exclusively in provisioning females who have evolved very high fidelity to their mates. Overall, females are not predicted to become completely faithful, but rather, the level of their faithfulness is expected to be controlled by a balance between selection for better genes (potentially supplied by top-ranked males) and better access for food and care (provided largely by low-ranked males).
Overall, my results confirm the theoretical plausibility of what has been viewed as a critical step in the evolution of our own species—the transition from promiscuity to strong pair-bonding. The model shows that such a sexual revolution could have been initiated by low-ranked males who started provisioning females to get matings; after the process got underway, it would lead to a kind of self-domestication, and the end result is a group-living species comprised of provisioning males and largely faithful females.
The results highlight the importance of considering the joint evolutionary dynamics of male and female traits. The model shows that nonrandom pair formation can have dramatic effects on evolutionary dynamics. The results emphasize the need for incorporating between-individual variation in theoretical and empirical studies of social dilemmas and behaviors; the commonly used simplifying assumption that individuals are identical can significantly bias the conclusions.
The models introduced and analyzed here assume that, initially, both sexes mate promiscuously. It is important to realize, however, that an underlying reason for the male’s dilemma as studied here is female promiscuity and the associated risks that the male’s investment in production might be stolen by other males. Therefore, some of my results may be relevant for polyandrous species. In polygynous species, male promiscuity may lead to another dilemma for males: whether to invest in obtaining more females or providing better provisioning and care to a smaller number of females. This other version of the male’s dilemma between production and appropriation is outside the scope of this work.
The importance of food-for-sex exchanges (5, 43, 51) in chimpanzees has been recently questioned in ref. 52, which argues that the benefits to females of food provisioned by males are small, whereas the role of female selectivity in determining male reproductive success is limited. Population genetic models tell us, however, that even weak evolutionary forces can result in dramatic phenotypic or behavioral changes if they act over multiple generations. A recent metaanalysis (53) shows correlation between male-to-female food transfer and the opportunity for female mate choice. There are some additional anatomical features of humans—bipedalism, hidden ovulation, and permanently enlarged mammary glands—that are easier to explain in terms of the pair-bonding model than the mate-guarding and communal breeding models (5, 54).
New paleontological data on 4.4-Myr-old fossils of Ardipithecus ramidis show that this species already had a reduced sexual size dimorphism and strong reduction in upper canine teeth (5). This finding and a loss of morphological adaptations to sperm competition in humans (5, 55) suggest that strong decline in the intensity of male-to-male conflict, which is one of the consequences of the transition to pair-bonding, happened soon after the hominins/chimpanzees divergence (5). If true, this finding has important implications for the theories of the runaway evolution of human brain size and intelligence over the past couple hundred thousand years. One controversial set of ideas (1, 2, 56–61) coming under the rubric of the “Machiavellian intelligence” or “social brain” hypothesis identifies selective forces resulting from within-group social competitive interactions as the most important factors in the evolution of hominids, who at some point in the past, became an ecologically dominant species (1, 2). These forces selected for more and more effective strategies (including deception, manipulation, alliance formation, exploitation of the expertise of others, etc.) of achieving social success and learning to use them. The social success translated into reproductive success (e.g., more children) (62, 63) selecting for larger and more complex brains. Pair-bonding would significantly decrease the efficiency of selection resulting from within-group competition for mating success. This effect would likely rule out within-group competition as a source of selection for larger brain size and intelligence. An intriguing alternative is selection resulting from between-group competitive and cooperative interactions.
The transition to strong pair-bonding opened a path to intensified male parental investment, which was a breakthrough adaptation with multiple anatomical, behavioral, and physiological consequences for early hominids and all of their descendants (4–6). The establishment of pair-bonding shifted competition between males for mates, which was potentially destructive for the group, to a new dimension which is beneficial for the group —competition to be a better provider to get better mates (64). Pair-bonding provided a foundation for the later emergence of the institution of modern family (65) as an outcome of additional processes, such as wealth accumulation and inheritance (66). Pair-bonding also made possible the recognition of male kin, dramatically expanding the efficiency of kin selection and helping by grandparents, leading to stronger within-group coalitions and alliances (67, 68), and allowing for subsequent evolution of widespread cooperation in general (6, 69).
Supplementary Material
Acknowledgments
I thank J. Auerbach, M. V. Flinn, M. Mesterton-Gibbons, K. Page, and reviewers for the comments on the manuscript.
Footnotes
The author declares no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1200717109/-/DCSupplemental.
References
- 1.Alexander RD. How Did Humans Evolve? Reflections on the Uniquely Unique Species. Ann Arbor, MI: Univ of Michigan, Museum of Zoology; 1990. [Google Scholar]
- 2.Flinn MV, Geary DC, Ward CV. Ecological dominance, social competition, and coalitionary arms races: Why humans evolved extraordinary intelligence? Evol Hum Behav. 2005;26:10–46. [Google Scholar]
- 3.Richerson PJ, Boyd R. Not by Genes Alone. How Culture Transformed Human Evolution. Chicago: Univ of Chicago Press; 2005. [Google Scholar]
- 4.Hrdy SB. Mothers and Others: The Evolutionary Origins of Mutual Understanding. Cambridge, MA: Belknap Press; 2011. [Google Scholar]
- 5.Lovejoy CO. Reexamining human origins in light of Ardipithecus ramidis. Science. 2009;326:74e1–74e8. [PubMed] [Google Scholar]
- 6.Chapais B. Primeval Kinship: How Pair-Bonding Gave Birth to Human Society. Cambridge, MA: Harvard Univ Press; 2008. [Google Scholar]
- 7.Chapais B. In: Mind the Gap. Tracing the Origins of Human Universals. Kappeler PM, Silk JB, editors. Heidelberg: Springer; 2010. pp. 19–51. [Google Scholar]
- 8.van Schaik CP, Burkart JM. In: Mind the Gap. Tracing the Origins of Human Universals. Kappeler PM, Silk JB, editors. Heidelberg: Springer; 2010. pp. 477–496. [Google Scholar]
- 9.Shultz S, Opie C, Atkinson QD. Stepwise evolution of stable sociality in primates. Nature. 2011;479:219–222. doi: 10.1038/nature10601. [DOI] [PubMed] [Google Scholar]
- 10.Hawkes K, Rogers AR, Charnov EL. The male’s dilemma: Increased offspring production is more paternity to steal. Evol Ecol. 1995;9:662–677. [Google Scholar]
- 11.Harada Y, Iwasa Y. Female mate preference to maximize paternal care: A two-step game. Am Nat. 1996;147:996–1027. doi: 10.1086/286125. [DOI] [PubMed] [Google Scholar]
- 12.Iwasa Y, Harada Y. Female mate preference to maximize paternal care. II. Female competition leads to monogamy. Am Nat. 1998;151:367–382. doi: 10.1086/286125. [DOI] [PubMed] [Google Scholar]
- 13.Kokko H, Morrell LJ. Mate guarding, male attractiveness, and paternity under social monogamy. Behav Ecol. 2005;16:724–731. [Google Scholar]
- 14.Rutberg AT. The evolution of monogamy in primates. J Theor Biol. 1983;104:93–112. doi: 10.1016/0022-5193(83)90403-4. [DOI] [PubMed] [Google Scholar]
- 15.de Waal FBM. Chimpanzee Politics: Power and Sex Among Apes. Baltimore: Johns Hopkins Univ Press; 2000. [Google Scholar]
- 16.Nishida T. Sexual behavior of adult male chimpanzees of the Mahale Mountains National Park. Primates. 1997;38:379–398. [Google Scholar]
- 17.Newton-Fisher NE. Hierarchy and social status in Budongo chimpanzees. Primates. 2004;45:81–87. doi: 10.1007/s10329-003-0064-6. [DOI] [PubMed] [Google Scholar]
- 18.Boesch C, Kohou G, Néné H, Vigilant L. Male competition and paternity in wild chimpanzees of the Taï forest. Am J Phys Anthropol. 2006;130:103–115. doi: 10.1002/ajpa.20341. [DOI] [PubMed] [Google Scholar]
- 19.Palombit R. Infanticide and the evolution of pair bonds in nonhuman primates. Evol Anthropol. 1999;7:117–129. [Google Scholar]
- 20.Brotherton P, Komers P. In: Monogamy: Mating Strategies and Partnerships in Birds, Humans and Other Mammals. Reichard U, Boesch C, editors. Cambridge, UK: Cambridge Univ Press; 2003. pp. 42–58. [Google Scholar]
- 21.Hirshleifer J. The paradox of power. Econ Polit. 1991;3:177–200. [Google Scholar]
- 22.Konrad K. Strategy and Dynamics in Contests. Oxford: Oxford Univ Press; 2009. [Google Scholar]
- 23.Frank S. Foundations of Social Evolution. Princeton: Princeton Univ Press; 1998. [Google Scholar]
- 24.Nowak M. Evolutionary Dynamics. Cambridge, MA: Harvard Univ Press; 2006. [Google Scholar]
- 25.McElreath R, Boyd R. Mathematical Models of Social Evolution. A Guide for the Perplexed. Chicago: Chicago Univ Press; 2007. [Google Scholar]
- 26.Reeve HK, Hölldobler B. The emergence of a superorganism through intergroup competition. Proc Natl Acad Sci USA. 2007;104:9736–9740. doi: 10.1073/pnas.0703466104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Crowley PH, Hwan Baik K. Variable valuations and voluntarism under group selection: An evolutionary public goods game. J Theor Biol. 2010;265:238–244. doi: 10.1016/j.jtbi.2010.05.005. [DOI] [PubMed] [Google Scholar]
- 28.Goodall J. The Chimpanzees of Gombe: Patterns of Behavior. Cambridge, MA: Belknap Press; 1986. [Google Scholar]
- 29.de Waal FB. Primates—a natural heritage of conflict resolution. Science. 2000;289:586–590. doi: 10.1126/science.289.5479.586. [DOI] [PubMed] [Google Scholar]
- 30.Hofbauer J, Sigmund K. Adaptive dynamics and evolutionary stability. Appl Math Lett. 1990;3:75–79. [Google Scholar]
- 31.Geritz SAH, Kisdi E, Meszéna G, Metz JAJ. Evolutionary singular strategies and the adaptive growth and branching of the evolutionary tree. Evol Ecol. 1998;12:35–57. [Google Scholar]
- 32.Waxman D, Gavrilets S. 20 questions on adaptive dynamics. J Evol Biol. 2005;18:1139–1154. doi: 10.1111/j.1420-9101.2005.00948.x. [DOI] [PubMed] [Google Scholar]
- 33.Mitani JC. Demographic influences on the behavior of chimpanzees. Primates. 2006;47:6–13. doi: 10.1007/s10329-005-0139-7. [DOI] [PubMed] [Google Scholar]
- 34.Hill KR, et al. Co-residence patterns in hunter-gatherer societies show unique human social structure. Science. 2011;331:1286–1289. doi: 10.1126/science.1199071. [DOI] [PubMed] [Google Scholar]
- 35.Barnard CJ, Sibly RM. Producers and scroungers—a general model and its implications to captive flocks of house sparrows. Anim Behav. 1981;29:543–550. [Google Scholar]
- 36.Barta Z, Giraldeau L-A. The effect of dominance hierarchy on the use of alternative foraging tactics: A phenotype-limited producing-scrounging game. Behav Ecol Sociobiol. 1998;42:217–223. [Google Scholar]
- 37.King AK, Isaac NJB, Cowlishaw G. Ecological, social, and reproductive factors shape producer-scrounger dynamics in baboons. Behav Ecol. 2009;20:1039–1049. [Google Scholar]
- 38.Mathot KJ, Giraldeau L-A. Within-group relatedness can lead to higher levels of exploitation: A model and empirical test. Behav Ecol. 2010;21:843–850. [Google Scholar]
- 39.Morin PA, et al. Kin selection, social structure, gene flow, and the evolution of chimpanzees. Science. 1994;265:1193–1201. doi: 10.1126/science.7915048. [DOI] [PubMed] [Google Scholar]
- 40.Copeland SR, et al. Strontium isotope evidence for landscape use by early hominins. Nature. 2011;474:76–78. doi: 10.1038/nature10149. [DOI] [PubMed] [Google Scholar]
- 41.Lalueza-Fox C, et al. Genetic evidence for patrilocal mating behavior among Neandertal groups. Proc Natl Acad Sci USA. 2011;108:250–253. doi: 10.1073/pnas.1011553108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hamilton WD. The genetical evolution of social behaviour. I. J Theor Biol. 1964;7:1–16. doi: 10.1016/0022-5193(64)90038-4. [DOI] [PubMed] [Google Scholar]
- 43.Gomes CM, Boesch C. Wild chimpanzees exchange meat for sex on a long-term basis. PLoS One. 2009;4:e5116. doi: 10.1371/journal.pone.0005116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Strassmann BI. Sexual selection, paternal care, and concealed ovulation in humans. Ethol Sociobiol. 1981;2:31–40. [Google Scholar]
- 45.Gross MR. Alternative reproductive strategies and tactics: Diversity within sexes. Trends Ecol Evol. 1996;11:92–98. doi: 10.1016/0169-5347(96)81050-0. [DOI] [PubMed] [Google Scholar]
- 46.Gagneux P, Boesch C, Woodruff DS. Female reproductive strategies, paternity and community structure in wild West African chimpanzees. Anim Behav. 1999;57:19–32. doi: 10.1006/anbe.1998.0972. [DOI] [PubMed] [Google Scholar]
- 47.Matsumoto-Oda A. Female choice in the opportunistic mating of wild chimpanzees (Pan troglodytes schweinfurthii) at Mahale. Behav Ecol Sociobiol. 1999;46:258–266. [Google Scholar]
- 48.Wrangham R. The evolution of sexuality in chimpanzees and bonobos. Hum Nat. 1993;4:47–79. doi: 10.1007/BF02734089. [DOI] [PubMed] [Google Scholar]
- 49.Lande R. Models of speciation by sexual selection on polygenic traits. Proc Natl Acad Sci USA. 1981;78:3721–3725. doi: 10.1073/pnas.78.6.3721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Gavrilets S. Fitness Landscapes and the Origin of Species. Princeton: Princeton Univ Press; 2004. [Google Scholar]
- 51.Stanford C. The hunting ecology of wild chimpanzees: Implications for the evolutionary ecology of pliocene hominids. Am Anthropol. 1996;98:96–113. [Google Scholar]
- 52.Gilby IC, Emery Thompson M, Ruane JD, Wrangham R. No evidence of short-term exchange of meat for sex among chimpanzees. J Hum Evol. 2010;59:44–53. doi: 10.1016/j.jhevol.2010.02.006. [DOI] [PubMed] [Google Scholar]
- 53.Jaeggi AV, van Schaik CP. The evolution of food sharing in primates. Behav Ecol Sociobiol. 2011;65:2125–2140. [Google Scholar]
- 54.Lovejoy CO. The origin of man. Science. 1981;211:341–350. doi: 10.1126/science.211.4480.341. [DOI] [PubMed] [Google Scholar]
- 55.McLean CY, et al. Human-specific loss of regulatory DNA and the evolution of human-specific traits. Nature. 2011;471:216–219. doi: 10.1038/nature09774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Byrne RW, Whiten A. Machiavellian Intelligence. Social Expertise and the Evolution of Intellect in Monkeys, Apes, and Humans. Oxford: Clarendon; 1988. [Google Scholar]
- 57.Whiten A, Byrne RW. Machiavellian Intelligence II. Extensions and Evaluations. Cambridge, UK: Cambridge Univ Press; 1997. [Google Scholar]
- 58.Dunbar RIM. The social brain hypothesis. Evol Anthropol. 1998;6:178–190. [Google Scholar]
- 59.Geary DC. The Origin of Mind. Evolution of Brain, Cognition, and General Intelligence. Washington, DC: American Psychological Association; 2005. [Google Scholar]
- 60.Roth G, Dicke U. Evolution of the brain and intelligence. Trends Cogn Sci. 2005;9:250–257. doi: 10.1016/j.tics.2005.03.005. [DOI] [PubMed] [Google Scholar]
- 61.Gavrilets S, Vose A. The dynamics of Machiavellian intelligence. Proc Natl Acad Sci USA. 2006;103:16823–16828. doi: 10.1073/pnas.0601428103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Betzig LL. Despotism and Differential Reproduction. A Darwinian View of History. New York: Albine Publishing Company; 1986. [Google Scholar]
- 63.Zerjal T, et al. The genetic legacy of the Mongols. Am J Hum Genet. 2003;71:717–721. doi: 10.1086/367774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Hawkes K. Why hunter-gatherers work. Curr Anthropol. 1993;34:341–361. [Google Scholar]
- 65.Engels F. The Origin of the Family, Private Property, and the State. Marx/Engels Selected Works. Vol 3. New York (1972, c1942): International Publishers; 1884. [Google Scholar]
- 66.Fortunato L, Archetti M. Evolution of monogamous marriage by maximization of inclusive fitness. J Evol Biol. 2010;23:149–156. doi: 10.1111/j.1420-9101.2009.01884.x. [DOI] [PubMed] [Google Scholar]
- 67.Gavrilets S, Duenez-Guzman EA, Vose MD. Dynamics of alliance formation and the egalitarian revolution. PLoS One. 2008;3:e3293. doi: 10.1371/journal.pone.0003293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Mesterton-Gibbons M, Gavrilets S, Gravner J, Akçay E. Models of coalition or alliance formation. J Theor Biol. 2011;274:187–204. doi: 10.1016/j.jtbi.2010.12.031. [DOI] [PubMed] [Google Scholar]
- 69.Boomsma JJ. Lifetime monogamy and the evolution of eusociality. Philos Trans R Soc Lond B Biol Sci. 2009;364:3191–3207. doi: 10.1098/rstb.2009.0101. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.