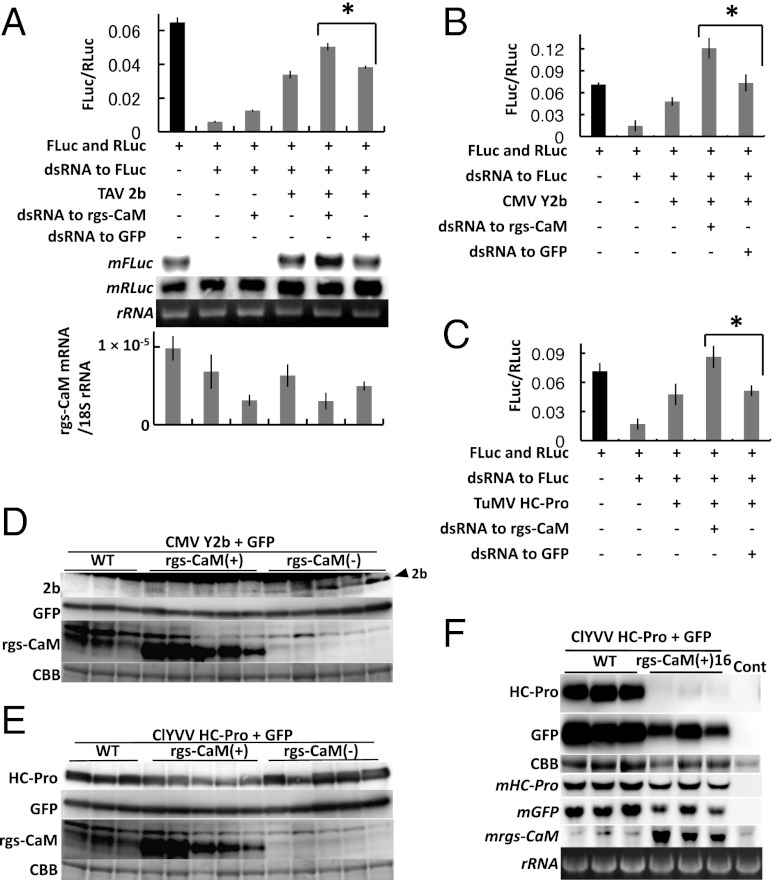
Fig. 2.
Effects of rgs-CaM–RSS interactions on the activity and stability of RSS proteins. (A–C) RSS activity of TAV 2b (A), CMV Y2b (B), and TuMV P1/HC-Pro (C) were measured by a dual luciferase assay in protoplasts of N. benthamiana. When endogenous rgs-CaM was silenced by a dsRNA cognate to rgs-CaM mRNA, RSS activities of all RSS proteins increased significantly, and even in the absence of RSS, RNAi activity was reduced (A; *P < 0.01, paired Student t test). Firefly and Renilla luciferase mRNAs (mFLuc and mRLuc, Middle) were also detected in RNA extracts from the same samples by Northern blotting (A, Middle), and results were consistent with the luciferase assay (A, Upper graph). The accumulation of endogenous rgs-CaM mRNA was monitored by real-time PCR to confirm its silencing (A, Lower graph). The x axis is the same as in Upper graph. Bars indicate SD. (D and E) CMV Y2b (D) or ClYVV P1/HC-Pro (E) and green fluorescent protein (GFP) were transiently expressed by agroinfiltration in transgenic T0 tobacco plants that either overexpressed rgs-CaM [rgs-CaM(+)] or silenced endogenous rgs-CaM [rgs-CaM(−)], and in nontransgenic plants (WT). Accumulated CMV 2b, ClYVV HC-Pro, GFP, and rgs-CaM proteins were detected by Western blotting. Arrowhead marks the 2b protein. Below each panel set, a Coomassie brilliant blue (CBB)-stained gel is shown as a loading control. Accumulation of the RSS proteins relative to GFP is shown in bar graphs in Fig. S4. (F) The same experiment shown in E was carried out using line 16 of T1 transgenic plants expressing rgs-CaM [rgs-CaM(+)16]. HC-Pro, GFP, and rgs-CaM mRNAs (mHC-Pro, mGFP, and mrgs-CaM) were additionally detected in total RNA extracts by Northern blotting. Lane control (Cont): Extracts from nontransgenic plant. Ribosomal RNA (rRNA) stained with ethidium bromide is shown as a loading control. We here note that the GFP mRNA levels were increased by the coinfiltrated HC-Pro in WT.