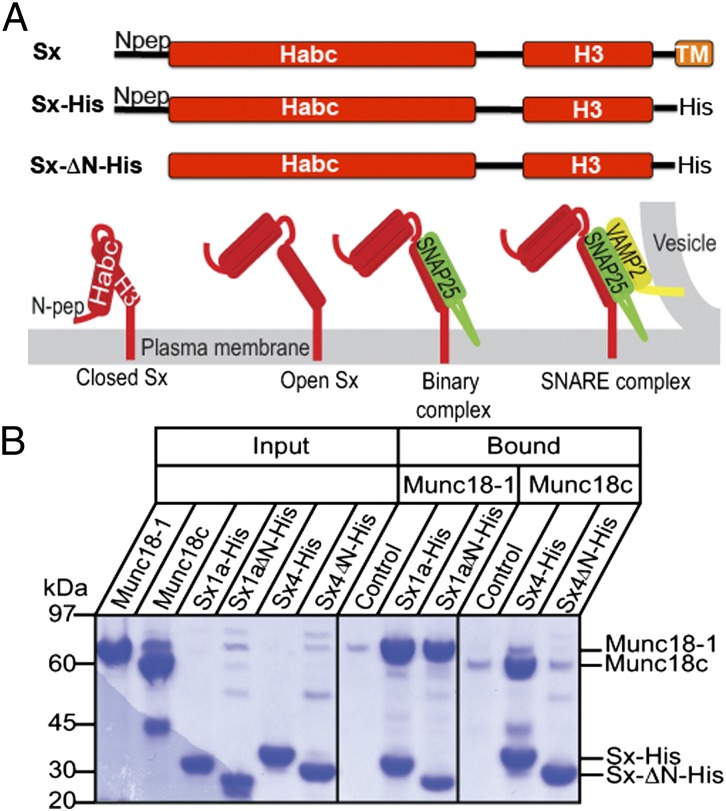
Fig. 1.
Sx constructs and interactions. We used Sx1a and Sx4 with the transmembrane anchor replaced with a polyhistidine tag (Sx-His) and with the N-peptide removed (SxΔN-His). (A) Schematic showing different forms of membrane-anchored Sx: closed Sx, open Sx, binary complex with SNARE partner SNAP (green), and ternary complex with SNARE partners [SNAP25 in green; VAMP2 (or synaptobrevin) in yellow]. The four-helix bundle of the SNARE ternary complex is required for membrane fusion, and open Sx is thought to be required to form SNARE complex. (B) Sx1a, Sx1aΔN, Sx4, and Sx4ΔN were immobilized on metal-affinity resin via the C-terminal polyhistidine tags and incubated with detagged Munc18-1 or Munc18c. The gels at right and center show the proteins that were pulled down (bound) on equivalent amounts of resin after extensive washing. Resin-bound Sx1a and Sx1aΔN are both able to pull down Munc18-1, whereas Sx4 but not Sx4ΔN pulls down Munc18c. (Left) Proteins used in the experiment. Control shows the negative control interaction of Munc18 with resin. These data are representative of three replicates.