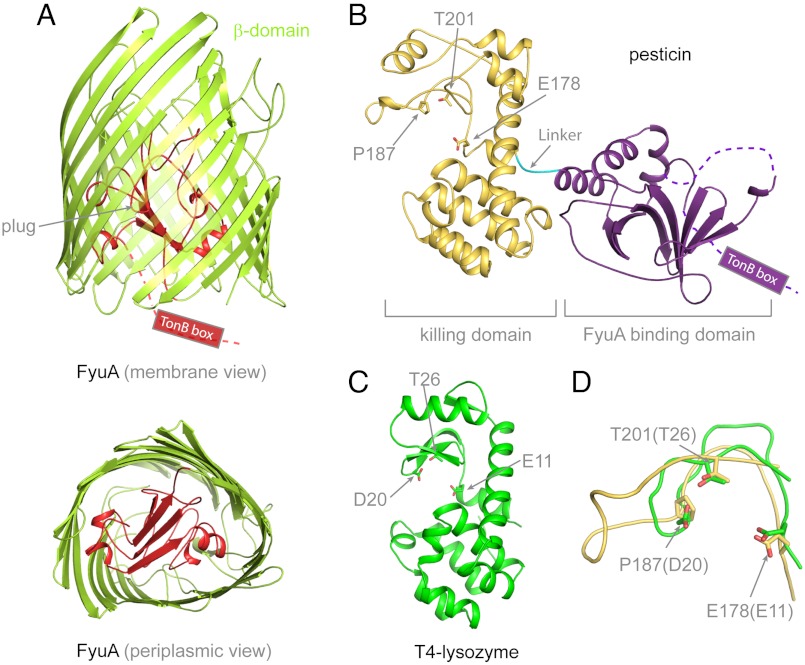
Fig. 1.
Crystal structures of Y. pestis FyuA and pesticin. (A) The crystal structure of FyuA consists of two domains: the β-barrel in green and the plug domain in red. The TonB box of FyuA was mostly disordered and not observed in the crystal structure. In the periplasmic view of FyuA, the location of the TonB box is not shown for clarity. (B) The crystal structure of pesticin consists of two distinct domains: The predominantly β-sheet N-terminal domain (FyuA binding domain) in purple and encompasses residues 1–164. The mainly α-helical C-terminal domain (residues 168–357) in gold (killing domain). A short linker connects the two domains and is shown in cyan that spans residues 165–167. The TonB box of pesticin was disordered and not observed in the crystal structure. The residues mutated in this study are labelled and shown in stick representation and include residues in the presumed active site of pesticin. (C) To illustrate that the C-terminal domain of pesticin is structurally similar to T4 lysozyme, the crystal structure of T4 lysozyme (green) (PDB code = 2LZM) is represented in the same orientation as the C-terminal domain of pesticin. Similarity between the two proteins is primarily structural; there is little sequence identity except for a partial conservation of active site residues that is apparent after a structural alignment. (D) Superimposition of the pesticin (gold) and T4 lysozyme (green) active sites shows that the catalytic threonine and glutamate residues are conserved in pesticin, whereas the catalytic aspartate in lysozyme is replaced by a proline in pesticin. Therefore, pesticin shares a functional resemblance to T4 lysozyme, but the active sites are not identical. For clarity, representation of secondary structure elements was omitted in D.